Mar 29 2017
 Nickel(II)-quinonoid complexes switch their colors and magnetism by absorbing or releasing methanol. (CREDIT - Kar P., Yoshida M., Shigeta Y., Usui A., Kobayashi A., Minamidate T., Matsunaga N., Kato M., Methanol-triggered vapochromism coupled with solid-state spin switching in a nickel(II)-quinonoid complex. Angewandte Chemie International Edition, January 23, 2017.)
Nickel(II)-quinonoid complexes switch their colors and magnetism by absorbing or releasing methanol. (CREDIT - Kar P., Yoshida M., Shigeta Y., Usui A., Kobayashi A., Minamidate T., Matsunaga N., Kato M., Methanol-triggered vapochromism coupled with solid-state spin switching in a nickel(II)-quinonoid complex. Angewandte Chemie International Edition, January 23, 2017.)
Researchers at Hokkaido University have successfully developed a nickel complex that alters magnetism and color when exposed to methanol vapor. The promising new material can be used as a chemical sensor as well as with future rewritable memory devices.
This phenomenon of color changes in a substance, stimulated by inorganic gas or volatile organic compound vapors, is known as vapochromism. This phenomenon was discovered towards the end of the last century, and since then scientists have focused their research on creating sensory materials that can visibly exhibit the presence of dangerous organic solvents. Moreover, study on materials that synchronize color and other property modifications, such as magnetism, could result in wider applications.
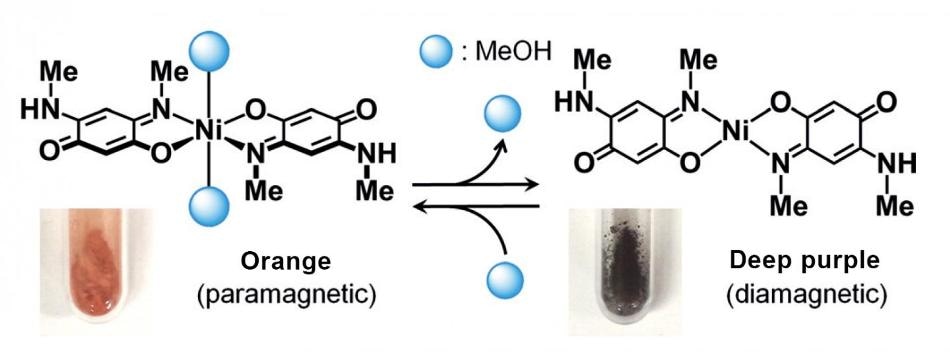
The team led by Masako Kato at Hokkaido University was keen on forming materials that concurrently change magnetism and color when exposed to vapors. Up to now, certain iron complexes were said to be capable of switching magnetism between "paramagnetism" and "diamagnetism" at room temperature, but typically fall into stable states when temperatures are lesser.
It was thus vital to create materials that can alter magnetism under a broader temperature range. The team chose a nickel(II)-quinonoid complex.
As the complex changes magnetism when its coordination structure changes, we hypothesized that if solvent vapors could bind to nickel ions directly, the complex would simultaneously change colors and magnetism.
Masako Kato, Hokkaido University
During their study, a nickel (II)-quinonoid complex was exposed to a high density methanol vapor environment. When methanol molecules bonded with the complex, the coordination structure was changed followed by an alteration in color from deep purple to orange. When exposed to vapors such as chloroform and ethanol, methanol molecules separated from the complex, and reversed its color to deep purple.
In partnership with Noriaki Matsunaga at Hokkaido University, they discovered that the color alterations happened along with alterations in magnetism. When methanol molecules were taken away from the complex, it changed from paramagnetism to diamagnetism state, and both states could be maintained under a broad temperature range.
This research marks the first time that vapor molecules have successfully changed magnetic states of nickel complexes. As the nickel complex reacts differentially to ethanol and methanol, it could be used in the future as a sensor material selective to methanol. We expect that further applications of this method may lead to novel materials that can record and erase data using vapor.
Masako Kato, Hokkaido University