Jul 26 2017
A light-responsive crystalline material capable of overcoming challenges encountered in earlier studies has been developed by Researchers at Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS) and the University of Tokyo.
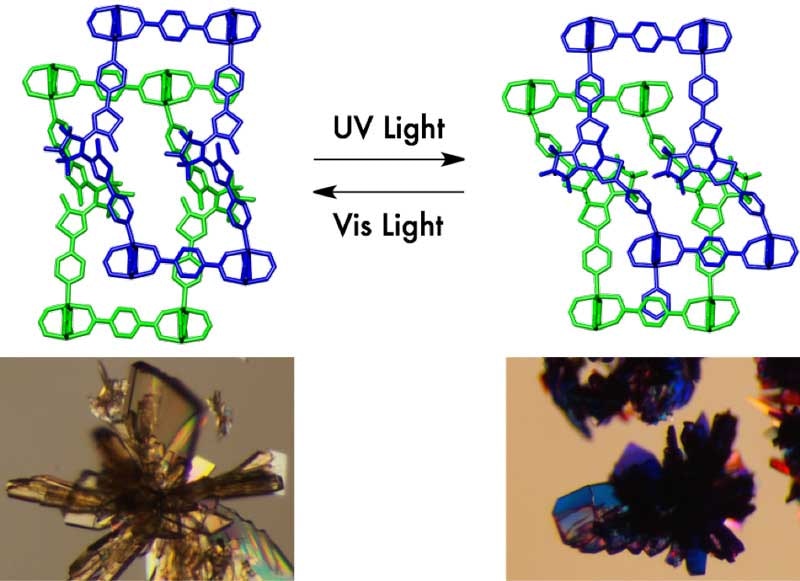 Entangled porous coordination polymers like ‘wire-and string puzzles’ enable reversible and repeatable photomodulation of CO2 sorption. The single crystals of the porous material showed a drastic color change upon irradiation of ultraviolet and visible light. Credit: KYOTO UNIVERSITY ICEMS
Entangled porous coordination polymers like ‘wire-and string puzzles’ enable reversible and repeatable photomodulation of CO2 sorption. The single crystals of the porous material showed a drastic color change upon irradiation of ultraviolet and visible light. Credit: KYOTO UNIVERSITY ICEMS
When exposed to light, photochromic molecules change their chemical structures or electronic states. These molecules play vital roles in the development of ‘photoresponsive’ materials capable of being used in delivery systems for controlled release of drugs, or to produce dynamic scaffolds for tissue engineering, among several other applications. However, to date, their use with solid materials has been established to be challenging since the materials have been too firm to allow reversible and repeatable changes.
Susumu Kitagawa of iCeMS, Hiroshi Sato of the University of Tokyo, along with their colleagues, developed a flexible porous crystal made up of a photoresponsive dithienylethene derivative, zinc ions (Zn2+), and 1,4-benenzenedicarboxylate.
The ‘porous coordination polymer’ comprised of two-dimensional sheets attached by pillars of photoresponsive molecules, which developed a three-dimensional, entangled framework. The entwined components are compared to twisted metal wire and string puzzles by the Researchers.
The channels changed shape when exposed to light because of the flexible nature of the entangled framework. When exposed to ultraviolet irradiation, the distance between the two layers shrank and when lit by visible light it expanded.
The potential of the material to uptake carbon dioxide (CO2) was tested by the Researchers. The material adsorbed up to 136 ml of CO2 when it was not irradiated. The pores shrank when exposed to ultraviolet light, reducing CO2 adsorption to 108 ml. CO2 absorption increased again to 129 ml when exposed to visible light. Re-exposure to ultraviolet light resulted in a decrease to 96 ml.
The entangled framework of the polymer allows these repeatable and reversible CO2 absorption changes; it makes room for the photoresponsive molecules to change while permitting them to discharge their strain into the flexible material.
Initial tests specified that the porous crystal is also capable of adsorbing other gases, such as nitrogen, at different temperatures, however an in depth analysis is needed.
“Our strategy will grant access to a new dimension of porous compounds as platforms for various photochemical conversions and the photomodulation of porous properties,” conclude the Researchers in their study, featured in the journal Nature Communications.