Aug 1 2017
A group of Penn State Researchers have experimentally demonstrated that an innovative, lightweight composite material has the ability to store energy in electric vehicles, flexible electronics, as well as aerospace applications at operating temperatures considerably greater than when compared to prevalent commercial polymers.
Moreover, the new polymer-based, ultrathin material can be synthesized using methods that are prevalent in the field of energy storage.
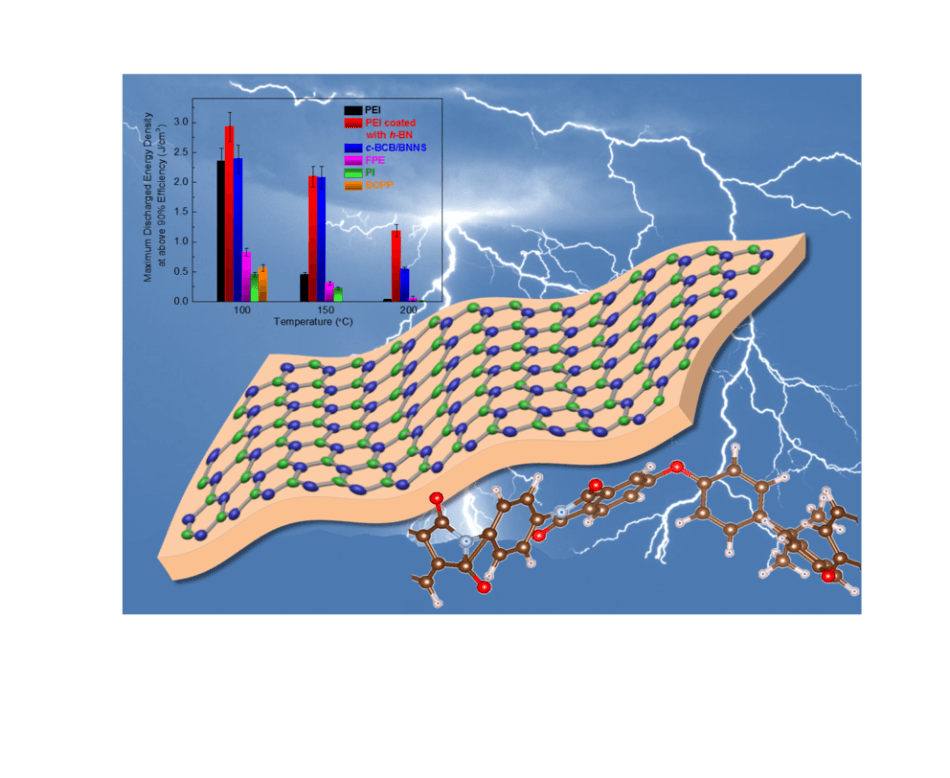 PEI coated with hexagonal boron nitride (hBN) nanosheets significantly outperforms competitive polymers at operating temperatures needed for electric vehicles and aerospace power applications. CREDIT: Feihua Liu/Penn State.
PEI coated with hexagonal boron nitride (hBN) nanosheets significantly outperforms competitive polymers at operating temperatures needed for electric vehicles and aerospace power applications. CREDIT: Feihua Liu/Penn State.
This is part of a series of work we have done in our lab on high-temperature dielectrics for use in capacitors. Prior to this work we had developed a composite of boron nitride nanosheets and dielectric polymers, but realized there were significant problems with scaling that material up economically.
Qing Wang, Professor of Materials Science and Engineering, Penn State
Scalability, that is, synthesizing improved materials in commercially appropriate quantities for devices, is an established difficulty in the case of various innovative, two-dimensional materials synthesized in academic labs.
“From a soft materials perspective, 2D materials are fascinating, but how to mass produce them is a question,” stated Wang. “Plus, being able to combine them with polymeric materials is a key feature for future flexible electronics applications and electronic devices.”
In order to overcome this difficulty, Wang’s lab worked in cooperation with a team from Penn State investigating two-dimensional crystals.
This work was conceived in conversations between my graduate student, Amin Azizi, and Dr. Wang’s graduate student, Matthew Gadinski. This is the first robust experiment in which a soft polymeric material and a hard 2D crystalline material have come together to create a functional dielectric device.
Nasim Alem, Assistant Professor of Materials Science and Engineering and a Faculty Member in Penn State’s Center for 2-Dimensional and Layered Materials
Azizi, who is at present a Post-doctoral Fellow at University of California-Berkeley, and Gadinski, who is a Senior Engineer at DOW Chemical, created a method in which chemical vapor deposition is employed to develop multi-layer, hexagonal boron-nitride nanocrystal films, where the films are transferred to both sides of a polyetherimide (PEI) film. Then, they secured the films to each other to form a three-layer sandwich structure by using pressure. The outcomes fascinated the Scientists as it was observed that pressure alone, without any chemical bonding, was adequate to synthesize a free-standing film durable enough to be prospectively produced by means of a high-throughput roll-to-roll procedure.
The outcomes of the research have been published in the latest issue of the Advanced Materials journal in a paper titled “High-performance Polymers Sandwiched with Chemical Vapor Deposited Hexagonal Boron Nitrides as Scalable High-Temperature Dielectric Materials.”
Hexagonal boron nitride is a material with high mechanical strength and a wide band-gap that makes it a better insulator and safeguards the PEI film against dielectric breakdown at higher temperatures, one of the main causes of failure in other polymer capacitors. At operating temperatures of greater than 176 °F, the prevalent finest commercial polymers start losing their effectiveness. In contrast, hexagonal-boron-nitride-coated PEI can function with better efficiency even above temperatures of 392 °F. The coated PEI was observed to be stable over 55,000 charge-discharge cycles under investigation even at greater temperatures.
Theoretically, all these high-performance polymers that are so commercially valuable can be coated with boron nanosheets to block charge injection. I think this will make this technology feasible for future commercialization.
Qing Wang, Professor of Materials Science and Engineering, Penn State
Alem further stated that “There are many devices made with 2D crystals at the laboratory scale, but defects make them a problem for manufacturing. With a large band-gap material like boron nitride, it does a good job despite small microstructural features that might not be ideal.”
First-principles computations showed that the electron barrier formed at the interface of the metal electrodes and the PEI/hexagonal boron-nitride structure and administered on the structure to supply current is considerably higher than conventional metal electrode-dielectric polymer contacts, thus restricting the injection of charges from the electrode into the film.
This study was carried out by the theoretical research team headed by Long-Qing Chen, Donald W. Hamer Professor of Materials Science and Engineering, Professor of Engineering Science and Mechanics, and Mathematics, Penn State.
Post-doctoral Scholar Qi Li and Graduate Student Feihua Liu in Wang’s lab; Undergraduate Mohammed Abu AlSaud from Alem’s lab; and Senior Scientist Jianjun Wang, Post-doctoral Scholar Yi Wang, and Graduate Student Bo Wang belonging to Chen’s team were the other contributors to this study.
This study was supported by the U.S. Office of Naval Research and the National Science Foundation.