Sep 26 2017
A fully scalable method to synthesize atom-precise platinum clusters has been developed by Scientists at Tokyo Institute of Technology (Tokyo Tech). These platinum clusters have potential use in catalytic applications.
The latest technique could pave the way for large-scale production of such atom-precise clusters. In order to demonstrate this method, the Scientists also performed the hydrogenation of styrene.
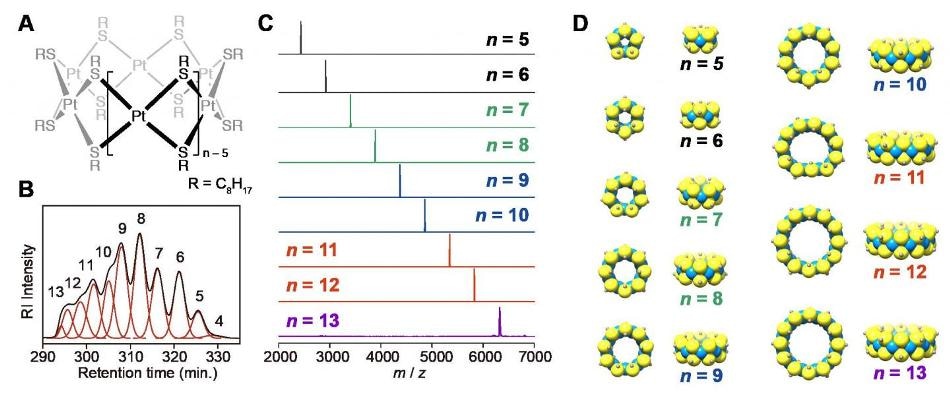 Figure (A) shows the chemical structure of the complexes, (B) shows the chromatogram of the HPLC separation with size exclusion columns, (C) shows the MALDI-TOF-mass spectra of the isolated platinum complexes measured with DCTB matrix, and (D) shows the optimized geometric structures with platinum as blue, sulfur as yellow, and α-carbon atoms in grey. Credit: Tokyo Institute of Technology
Figure (A) shows the chemical structure of the complexes, (B) shows the chromatogram of the HPLC separation with size exclusion columns, (C) shows the MALDI-TOF-mass spectra of the isolated platinum complexes measured with DCTB matrix, and (D) shows the optimized geometric structures with platinum as blue, sulfur as yellow, and α-carbon atoms in grey. Credit: Tokyo Institute of Technology
These subnanometer noble-metal clusters have already been analyzed for these possibilities in various catalytic applications. For instance, platinum is traditionally used as a catalyst for a fuel cell, which is not only conductive but also displays magnetism. While platinum material is essential for advanced energy grid, it is the "rare metal" whose reserves are rather restricted. In order to use the resources effectively, the atomic level accuracy of subnanometer metal clusters should be improved and the amount of synthesis should be increased.
Although many different methods of synthesis have been developed from stable ligand-protected "magic number" clusters or multi-nuclear metal complexes, the actual preservation of atomicity was never provided and the precursors were not scalable. This may have been partly due to the high metal-to-ligand binding energy that needs very high calcination temperature, which leads to the aggregation of clusters.
Researchers at Tokyo Tech studied platinum-thiolates as they may cause the formation of bare metal clusters via reductive metal-sulfur bond cleavage and these materials also form tiara-like complexes that are sufficiently stable for isolation.
Kimihisa Yamamoto, Takane Imaoka and co-workers investigated the monodispersed subnanometer platinum clusters as well as their catalytic activity for preparative-scale reactions via non-destructive conversion from platinum thiolate complexes to platinum tiara-like clusters. But, before this study, just one example of a platinum thiolate tiara-like complex with complete chemical identification has been reported so far.
As a basis for this research, the Scientists first investigated the fundamental reactions between n-octanethiol and PtCl4. The outcomes of these basic reactions indicated that platinum thiolates first experience a linear chain growth and this is followed by the entropically favorable formation of tinier cyclic compounds. Depending on these results, the team optimized the synthesis process in two steps that led to a crude product with platinum tiara-like complexes of different ring sizes.
The resultant platinum tiara-like complexes were additionally isolated into their pure clusters on the basis of ring sizes via size exclusion chromatography depending on preparative recycling HPLC. The catalytic activity and thermostability of the platinum tiara-like complexes were then studied to confirm whether these complexes can be used as precursors for atom-precise platinum clusters.
The team ultimately found that the tiara-like platinum thiolate complexes can be used as precursors in the synthesis of monodispersed zero-valent platinum clusters with a particular atomicity: a reaction that could not be realized by earlier chemical techniques. This is the first approach to reveal a preparative-scale reaction using atom-precise platinum clusters with a single digit atomicity, which may be useful for developing high-performance catalysts.
More studies on the support and stability effect are required to gain a complete insight into the catalyst system through such small-sized clusters.