Sep 27 2017
As part of the race to replace silicon in low-cost solar cells, semiconductors called metal halide perovskites are favored as they can be solution-processed into thin films with exceptional photovoltaic efficiency. A partnership between KAUST and Oxford University Researchers has presently uncovered a strategy that involves growing perovskites into centimeter-scale, highly pure crystals, thanks to the influence of surface tension.
 In-depth analysis of the mechanisms that generate floating crystals from hot liquids could lead to large-scale, printable solar cells Reproduced with permission from reference 1© 2017 American Chemical Society
In-depth analysis of the mechanisms that generate floating crystals from hot liquids could lead to large-scale, printable solar cells Reproduced with permission from reference 1© 2017 American Chemical Society
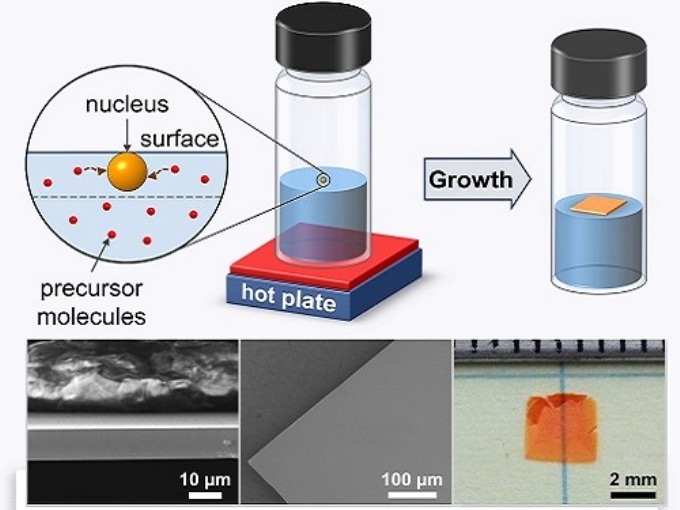
In their natural state, perovskites have problems in moving solar-generated electricity as they crystallize with randomly oriented grains. Osman Bakr from KAUST’s Solar Center and co-workers are working on discovering ways that will help to dramatically speed up the flow of these charge carriers using inverse temperature crystallization (ITC). This technique employs special organic liquids and thermal energy in order to force perovskites to solidify into structures similar to single crystals—the optimal arrangements for device purposes.
While ITC creates superior-quality perovskites far faster than standard chemical methods, the probing mechanisms that start crystallization in hot organic liquids are poorly understood. Ayan Zhumekenov, a PhD student in Bakr’s group, recalls spotting a significant piece of evidence during efforts carried out for adapting ITC toward large-scale manufacturing.
At some point, we realized that when crystals appeared, it was usually at the solution’s surface,” he says. “And this was particularly true when we used concentrated solutions.
Ayan Zhumekenov, PhD student, Bakr’s group
The KAUST team combined with Oxford theoreticians in order to identify how interfaces influence the growth of perovskite in ITC. They suggest that metal halides and solvent molecules originally cling together in tight complexes that start to stretch and weaken at greater temperatures. The complex breaks and perovskites begin to crystallize with sufficient thermal energy.
However interestingly, the Researchers discovered that complexes positioned at the solution surface can go through additional forces because of surface tension — the strong cohesive forces that enable specific insects to stride over ponds and lakes. The extra pull provided by the surface makes it a lot easier to detach the nucleate crystals and solvent-perovskite complexes that float on top of the liquid.
By exploiting this knowledge, the Researchers were able to produce centimeter-sized, ultrathin single crystals and prototype a photodetector with characteristics comparable to modern devices. Even though the single crystals are presently fragile and tough to handle due to their microscale thicknesses, Zhumekenov explains that this method could help in directing the perovskite growth onto particular substrates.
Taking into account the roles of interfaces and surface tension could have a fundamental impact, we can get large-area growth, and it’s not limited to specific metal cations—you could have a library of materials with perovskite structures.
Ayan Zhumekenov, PhD student, Bakr’s group