Nov 20 2017
A finding by an international group of researchers from Princeton University, the Georgia Institute of Technology and Humboldt University in Berlin directs the way to a more extensive application of an advanced technology commonly known as organic electronics.
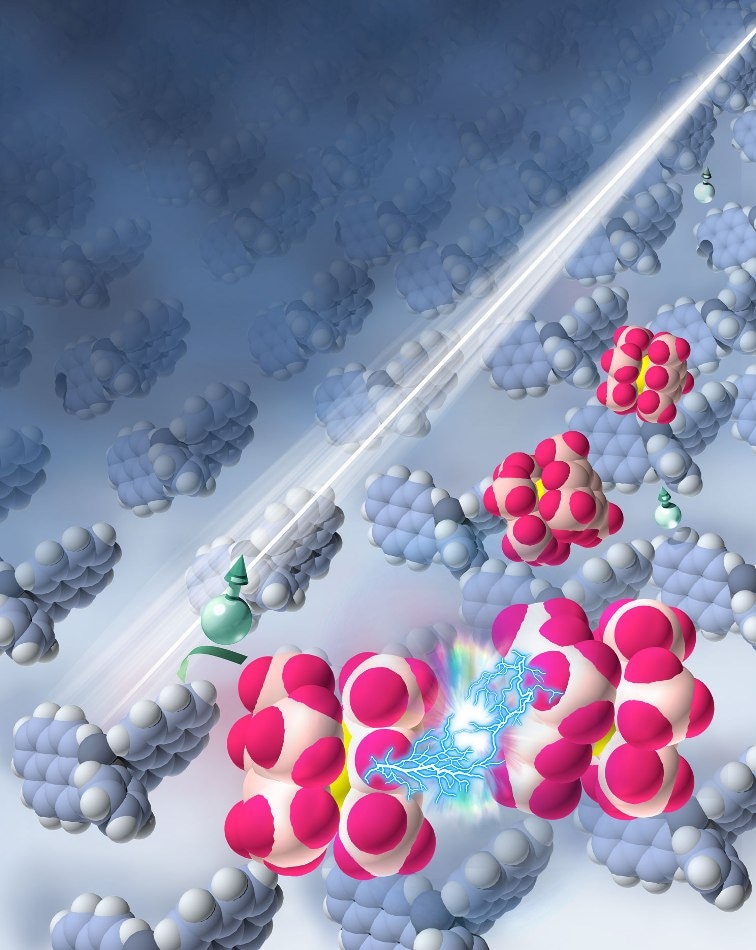 Researchers used UV light to excite molecules in a semiconductor, triggering reactions that split up and activated a dopant (illustration by Jing Wang and Xin Lin)
Researchers used UV light to excite molecules in a semiconductor, triggering reactions that split up and activated a dopant (illustration by Jing Wang and Xin Lin)
The research, published on the 13th of November in the Nature Materials journal, concentrates on organic semiconductors, a group of materials valued for their applications in upcoming technologies such as solar energy conversion, flexible electronics, and high-quality color displays for smartphones and televisions. In the short term, the progress should predominantly help with organic LEDs that function at high energy to emit colours such as blue and green.
Organic semiconductors are ideal materials for the fabrication of mechanically flexible devices with energy-saving low temperature processes, one of their major disadvantages has been their relatively poor electrical conductivity, which leads to inefficient devices with a shorter operating lifetime than is required for commercial applications. We are working to improve the electrical properties of organic semiconductors to make them available for more applications.
Xin Lin, a doctoral student and a member of the Princeton research team.
Semiconductors, usually made of silicon, are the basis of advanced electronics because engineers can exploit their unique properties to regulate electrical currents. Among numerous applications, semiconductor devices are used for signal amplification, computing, and switching. They are used in energy-saving devices such as LEDs and devices that convert energy such as solar cells.
Vital to these functionalities is a process known as doping, in which the semiconductor’s chemical structure is adjusted by incorporating a small quantity of chemicals or impurities. By carefully selecting the type and quantity of dopant, researchers can modify semiconductors’ electronic structure and electrical behavior in a range of ways.
In their newest Nature Materials article, the researchers explain a new approach for significantly raising the conductivity of organic semiconductors, which are formed of carbon-based molecules instead of silicon atoms. The dopant, a ruthenium-containing compound, is a reducing agent, which means it gains electrons in the organic semiconductor as portion of the doping process. The addition of the electrons is crucial to boosting the semiconductor’s conductivity. The compound belongs to a recently-introduced group of dopants called dimeric organometallic dopants. In contrast to many other robust reducing agents, these dopants are stable when exposed to air but still function as powerful electron donors both in solid state and in solution.
Seth Marder and Steve Barlow from Georgia Tech, who guided the development of the new dopant, referred to the ruthenium compound a “hyper-reducing dopant.” They said it is odd, not only its combination of air stability and electron donation strength, but in its ability to function with a group of organic semiconductors that have earlier been very hard to dope. In research conducted at Princeton, the team discovered that the new dopant boosted the conductivity of these semiconductors nearly a million times.
The ruthenium compound is a dimer, which means it comprises of two identical molecules, or monomers, linked by a chemical bond. As is, the compound is comparatively stable and, when incorporated to these difficult-to-dope semiconductors, it does not react and stays in its equilibrium state. That posed a problem because in order to raise the conductivity of the organic semiconductor, the ruthenium dimer has to split and discharge its two identical monomers.
Lin, the Princeton doctoral student who was lead author of the Nature Materials article, said the researchers sought for various ways to break up the ruthenium dimer and trigger the doping. Ultimately, he and Berthold Wegner, a visiting graduate student from the group of Norbert Koch at Humboldt University, hit upon integrating energy by irradiating it with UV light, which efficiently excited molecules in the semiconductor and started the reaction. When exposed to the light, the dimers divided into monomers, and the conductivity increased.
Following that, the researchers made an appealing observation.
“Once the light is turned off, one might naively expect the reverse reaction to occur” and the amplified conductivity to disappear, Marder said in an email. “However, this is not the case.”
The researchers discovered that the ruthenium monomers stayed isolated in the semiconductor _ increasing conductivity _ even though thermodynamics should return the molecules to their initial configuration as dimers. Antoine Kahn, a Princeton professor who guides the research team, said the physical layout of the molecules inside the doped semiconductor offers a probable answer to this puzzle. The hypothesis is that the monomers are spread in the semiconductor in such a way that it is extremely hard for them to return to their original configuration and re-form the ruthenium dimer. To reform, he said, the monomers should be facing in the correct orientation, but in the mixture they remain aakew. So, even though thermodynamics indicates that dimers should reform, the majority of them never snap back together.
The question is why aren’t these things moving back together into equilibrium, the answer is they are kinetically trapped.
Antoine Kahn, the Stephen C. Macaleer '63 Professor in Engineering and Applied Science.
In fact, the researchers watched the doped semiconductor for more than a year and found very minimal decrease in the electrical conductivity. Moreover, by observing the material in LEDs created by the group of Barry Rand, an assistant professor of electrical engineering at Princeton and the Andlinger Center for Energy and the Environment, the researchers found that doping was constantly re-activated by the light generated by the device.
The light triggers the system more, which results in more light production and more activation until the system is completely activated, Marder said. “This alone is a novel and surprising observation.”
Other paper’s authors include: graduate students Kyung Min Lee, Michael A. Fusella, and Fengyu Zhang, of Princeton, and Karttikay Moudgil of the Georgia Institute of Technology
The research was supported partly by the National Science Foundation and the U.S. Department of Energy.