Jan 25 2018
While there is an emerging market for organic solar cells – they comprise of materials that are more abundant, cheaper and more environmentally friendly than those employed in typical solar panels – they also tend to be less efficient in transforming sunlight to electricity than standard solar cells.
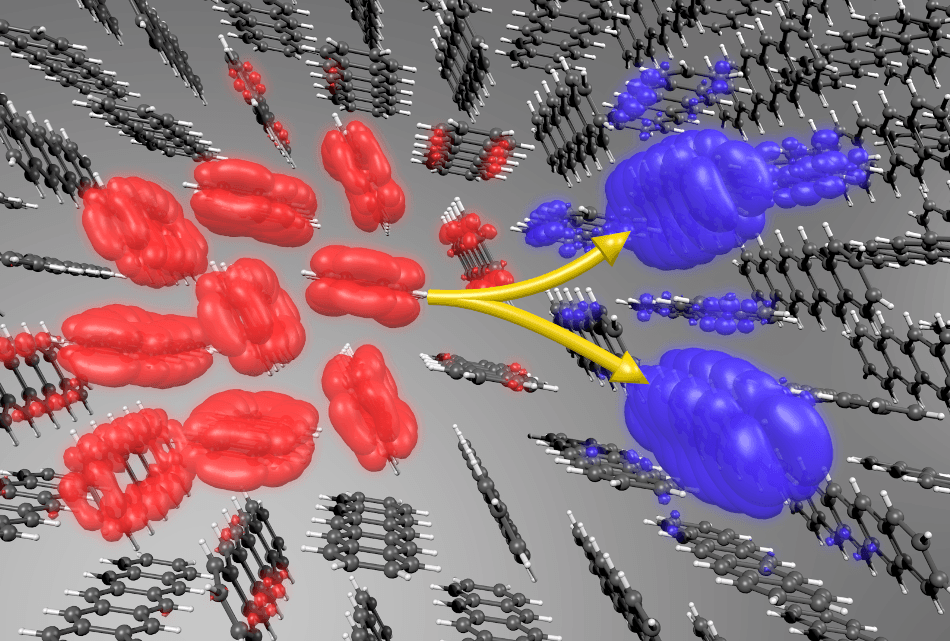 In this image, an optically excited spin-singlet state (red - featuring electron-hole pairs) splits into a pair of spin-triplet states (blue). The individual triplets have equal and opposite center-of-mass momenta – they behave like waves moving in opposite directions along a crystal. The gray and white spheres represent carbon and hydrogen atoms, respectively. (Image credit: Florian Brown-Altvater/Berkeley Lab)
In this image, an optically excited spin-singlet state (red - featuring electron-hole pairs) splits into a pair of spin-triplet states (blue). The individual triplets have equal and opposite center-of-mass momenta – they behave like waves moving in opposite directions along a crystal. The gray and white spheres represent carbon and hydrogen atoms, respectively. (Image credit: Florian Brown-Altvater/Berkeley Lab)
Recently, scientists who are members of the Center for Computational Study of Excited-State Phenomena in Energy Materials (C2SEPEM), a new energy materials-related science center located at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab), succeeded in solving a mystery that could result in efficiency gains.
They highlighted the source of an efficient and ultrafast process that spawns a number of carriers of electrical charge from one particle of light in organic crystals, which are considered to be integral to this greatly popular form of solar cells.
This process – known as “singlet fission”, since it is akin to the splitting of atomic nuclei in nuclear fission, to produce two lighter atoms from a heavier one – proves to be promising for dramatically improving the efficiency of organic solar cells by quickly transforming more of sunlight’s energy into electrical charges instead of just losing it to heat.
The research team discovered a new mechanism describing how this reaction can take place within just tens of femtoseconds (quadrillionths of a second), before various other competing effects can steal away their energy. Their study has been featured in the Dec. 29 issue of the journal Physical Review Letters.
“We actually discovered a new mechanism that allows us to try to design better materials,” said Steven G. Louie, director of C2SEPEM, a DOE-supported center that includes researchers from Berkeley Lab; the University of California, Los Angeles; the University of Texas at Austin; and the Georgia Institute of Technology. Louie, a co-leader of the study, is also a senior faculty scientist in Berkeley Lab’s Materials Sciences Division and a professor of physics at UC Berkeley.
C2SEPEM concentrates on developing methods, theories, and software in order to assist in explaining difficult processes in energy-related materials.
In the splitting process, a composite particle made up of an electron, which comprises of a negative charge, and its partner hole – an empty electron position in a material’s atomic structure that acts like a particle by carrying a positive charge – rapidly transforms into two electron-hole pairs. This doubles the charge-carrying potential in the material while preventing the loss of energy in the form of heat.
There’s a lot we still don’t understand about the fundamental physics of this process in crystalline materials that we are hoping to shed more light on.
The computational method that we developed is very predictive, and we used it to understand singlet fission in a new way that may allow us to design materials even more efficient at harvesting light, for example.
Jeffrey B. Neaton, Associate Director of C2SEPEM
Neaton is also the Associate Laboratory Director for Energy Sciences at Berkeley Lab, the director of Berkeley Lab’s Molecular Foundry, and a physics professor at UC Berkeley.
Louie considered the fact that a number of past efforts had concentrated on only a few molecules inside the material – in this situation, the crystallized form of pentacene, which is made up of carbon and hydrogen – to learn about these exotic effects. But of these approaches could have oversimplified the effects driving singlet fission.
“There have been many theoretical efforts to try to understand what’s going on,” he said.
In this recent study, the research team started with a large-scale view of the whole structure of the crystallized pentacene and it's symmetry – the repeating patterns in its atomic framework.
“It’s like trying to explain the ocean by either looking at it molecule by molecule, or looking at a whole wave,” said Felipe H. da Jornada, a co-lead author of the study with Sivan Refaely-Abramson. Both are affiliated with C2SEPEM besides being postdoctoral researchers at Berkeley Lab and UC Berkeley.
“Our approach directly captures the whole crystal,” no matter the size, he noted.
The team made use of calculations carried out partially at Berkeley Lab’s Molecular Foundry, and supercomputing resources at the Lab’s National Energy Research Scientific Computing Center in order to develop, model, and then test their new theories of the fission process.
“We believe these theories can also be applied to very different materials,” said Refaely-Abramson, “and in this sense, theory is very important.” Earlier experiments had missed some of the vital clues about the crystal structure’s role in the singlet fission mechanism.
The study draws to conclusion that in order to efficiently double these electron-hole pairs, the sampled material will have display a particular kind of symmetry, or repeated combinations of molecules, inside its crystal structure – just as a room’s floor is capable of displaying a multitude of simple, repeating patterns employing the same tiles.
The effectiveness of the singlet fission process appears to depend greatly on the number of molecules packed inside each repeating pattern or “motif” in the crystal, and on a specific type of symmetry in which there is a 180-degree rotation and mirroring of these motifs. This relationship between efficiency and symmetry, the researchers discovered, permits them to make powerful predictions on the efficiency of the complete fission.
Those predictions can be possible, though, if the electron-hole pairs in the sample act as wavelike objects passing all over the whole crystal, just like waves in an ocean. This approach also provided them new insight concerning the splitting process, and how the recently developed pairs must act like waves spreading in opposite directions.
Still, there are a number of steps that must be worked out in order to make these findings more applicable to real-world applications, the researchers highlighted. In solar cells, for instance, electrons must be competently liberated from their pairing with holes in order to harvest their energy and enhance solar panel performance.
Comprehending the doubling of charge carriers in a material could enable researchers to better explain and also engineer reverse processes, too – such as the technology employed in a few mobile phone displays that decreases the number of charge carriers (a process called triplet fusion), said Neaton.
Louie considered that the multidisciplinary team that was gathered for the study, a vital aspect of the C2SEPEM center, indeed played a vital role in launching new thinking in order to address a decades-old problem.“This is one of the first important topics that we could address, and now it’s come to fruition,” he said.
The Molecular Foundry and NERSC are both DOE Office of Science User Facilities.
The U.S. Department of Energy’s Office of Science supported this work.