Apr 18 2018
Annually, the impacts of corroding materials cost the global economy over $1 trillion. Some alloys are exposed to extreme temperatures and stress, causing the formation of an oxide film, which in turn accelerates the breakdown of the alloys.
The exact reasons that make these high-stress and high-temperature conditions so favorable for corrosion are unclear, particularly in microelectromechanical devices. Chinese researchers have begun to work toward understanding the causes for corrosion of materials under mechanical stress. They have reported their study in the Journal of Applied Physics, from AIP Publishing.
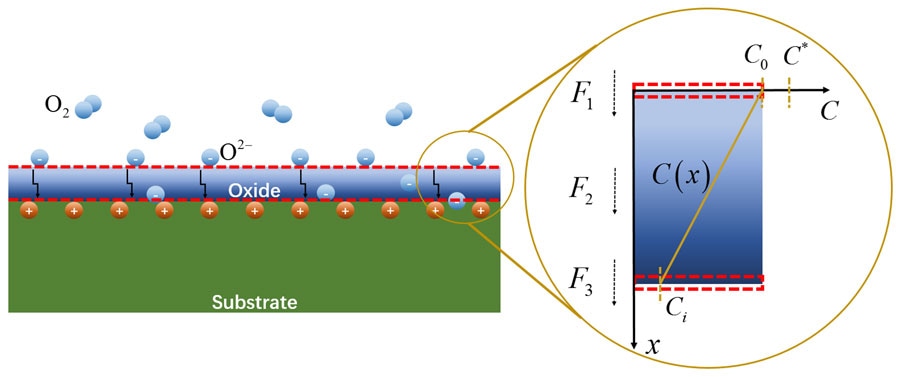 Schematic of an oxide film/substrate system and the oxidation process. In the first stage, the flux affects the diffusion and adsorption of oxygen from gas to the gas/oxide interface. (Credit: Mengkun Yue)
Schematic of an oxide film/substrate system and the oxidation process. In the first stage, the flux affects the diffusion and adsorption of oxygen from gas to the gas/oxide interface. (Credit: Mengkun Yue)
A research group led by Professor Xue Feng of Tsinghua University has explained the effect of mechanical stress on the oxidation process. Their model makes use of oxidation kinetics to describe how stress alters chemical reactions at interfaces and cause oxidation, and how stress affects the oxidation species that diffuse all over the oxide layer.
“Our work is in the direction of fundamental research, but it is indeed based on engineering problems,” Feng noted. “We expect that it provides guidelines for more accurate predictions in engineering applications, including better designs to compensate for material and system failure by taking into account the oxidation process.”
For several years, the focus of the study of the chemomechanical coupling of oxidation and physical stress was on associating stress to one of two different attributes of alloy corrosion. Particularly, stress contributes to accelerating the oxidation taking place on the surface of the material at the interface between the device and oxygen that is present in the surrounding air. Moreover, stress modifies the manner in which oxidative compounds diffuse all through a material’s nanoscale structure.
The research by the team combines the oxidation process and stress into a new model. First, a substrate, which is usually the corroding alloy, forms a metal oxide layer by absorbing oxygen. This layer allows more oxygen to diffuse through it, which can react with the subsequent alloy layer behind the oxidation interface.
“Our work here mainly deals with the second and third stages, in which the stress, either externally applied mechanical loading or intrinsically generated stress due to the oxide formation itself, could affect the diffusion and chemical reaction process,” added Mengkun Yue from Tsinghua University, another author of the paper.
The model presented by the group predicted that, on compression, materials under heavy loads absorb less oxygen. Likewise, the stresses pulling the material apart create additional space for oxygen to permeate the alloy.
The researchers tested this framework on samples of SiO2 grown on a Si substrate with the help of multibeam interferometry. This method had been formerly demonstrated by other researchers, and the theoretical predictions were consistent with the data.
Xufei Fang, an author on the paper at Max Planck Institute for Iron Research, expects that the verification of a unified model for stress-oxidation coupling can facilitate enhancements of microelectromechanical devices. Under stress or at elevated temperatures, it is possible for these devices to go through significantly more oxidation owing to their large surface-area-to-volume ratio.
“We expect a more general application of our model and we will develop our model further, in the next steps, to apply them to microscale systems,” Fang added.