May 3 2018
A number of beneficial substances - for instance, pharmaceuticals, dyes, and advanced materials- on which our daily life is dependent, are produced by organic chemistry. Yet, it is extremely challenging to predict the characteristics of chemical substances.
This is exactly why chemists must produce thousands of test molecules to find the one that is apt. At present, rapid production of these substances is accomplished by using catalysts, which put together multiple molecular fragments into a single complex structure. In 2010, the significance of such catalysts was recognized by the Nobel prize.
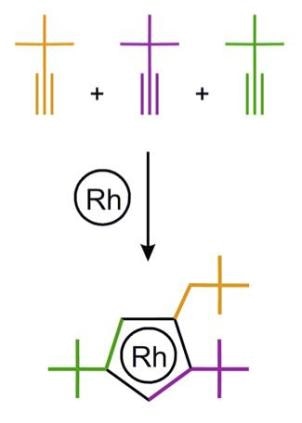 The active center of the catalyst, the rhodium atom, is “wearing” an asymmetric “hat,” which was constructed from three acetylene molecules. (Image credit: Dmitry S. Perekalin)
The active center of the catalyst, the rhodium atom, is “wearing” an asymmetric “hat,” which was constructed from three acetylene molecules. (Image credit: Dmitry S. Perekalin)
Recently, a group of Russian chemists headed by Professor Dmitry Perekalin from Nesmeyanov Institute of Organoelement Compounds has created an innovative rhodium catalyst for organic synthesis. The rhodium atom, which is the active center of the catalyst, “wears” an asymmetric “hat,” which was built using three acetylene molecules. The molecules with right and left “hats” (i.e. mirror images of each other) were isolated from each other with a natural “left-handed” amino acid proline. The asymmetry of this “hat” enables the catalyst to put together the reacting molecules with complete spatial control. The technique is anticipated to assist in developing innovative antifungal agents for the agricultural industry.
The newly found catalyst activates a carbon-hydrogen bond in the aromatic acid derivatives and combines them with alkene molecules. This process leads to the conversion of monocyclic molecules into tricyclic ones in one step. Molecular structures are made more rigid by fusion of a number of rings; hence, they possibly fit into biochemical “locks” of natural enzymes as “a key”. Due to the fact that both alkene and aromatic acid components can be varied, the reaction can easily produce 2500 probable “keys” from 100 initial reactants.
Studies into such asymmetric catalyst were begun by scientists from Switzerland (Professor Cramer from Lausanne and Professor Ward from Basel) and also the United States (Professor Rovis from New York); however, at present, researchers from Germany, China, and Russia have joined the study. The challenge at present is to make the catalysts less expensive by substituting the costly rhodium metal with less expensive ruthenium or even cobalt.
Mainstream research tends to make catalysts more and more complex. Sometimes it goes beyond common sense and the catalyst becomes more difficult to make than the reaction, which it is supposed to help with. Our idea was to go in the opposite direction - to make things as simple as possible. And this is one of the main reasons why we have discovered something new.
Professor Perekalin