Jun 7 2018
Scientists have shown how a novel type of transparent conductive electrode film based on nanopatterned silver can be fabricated on large scale.
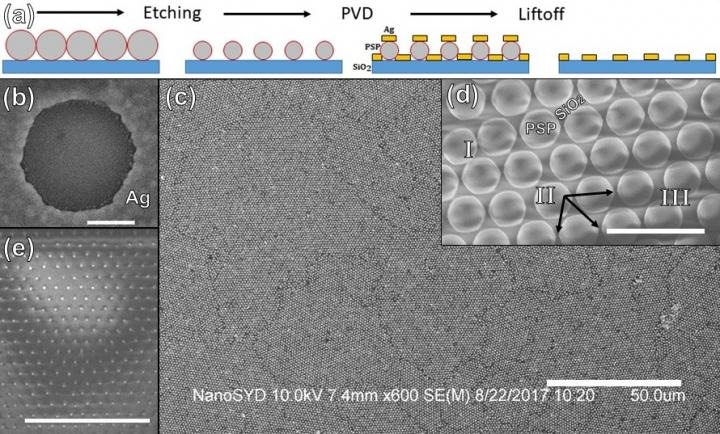 The researchers used colloidal lithography was used to create a thin film that was transparent and conductive thin film. (a) Schematic illustration of the fabrication process. (b) A single nanohole after the silver was deposited deposition and dissolving of the plastic particle. Scale bar: 200 nm. (c) Low magnification micrograph of deposited silver thin-film on homogenous particle monolayer, demonstrating large-scale feasibility. Scale bar: 50 microns. (d) Particle monolayer on substrate after spin-coating and a short (60 s) time in the plasma oven: Scale bar: 2 microns. (e) Particle monolayer after a long (3 min) time in the plasma oven, demonstrating that original particle positions are preserved even after significant size reduction. Scale bar: 10 microns. (Image credit: Jes Linnet, University of Southern Denmark)
The researchers used colloidal lithography was used to create a thin film that was transparent and conductive thin film. (a) Schematic illustration of the fabrication process. (b) A single nanohole after the silver was deposited deposition and dissolving of the plastic particle. Scale bar: 200 nm. (c) Low magnification micrograph of deposited silver thin-film on homogenous particle monolayer, demonstrating large-scale feasibility. Scale bar: 50 microns. (d) Particle monolayer on substrate after spin-coating and a short (60 s) time in the plasma oven: Scale bar: 2 microns. (e) Particle monolayer after a long (3 min) time in the plasma oven, demonstrating that original particle positions are preserved even after significant size reduction. Scale bar: 10 microns. (Image credit: Jes Linnet, University of Southern Denmark)
Transparent electrodes are used by flat panel televisions and smartphone touch screens to detect touch as well as to rapidly change the color of each pixel. In addition to being less brittle, silver is more chemically resistant when compared to materials being used to develop these electrodes. As a result, the novel films could provide a long-lasting and high-performance option for use with flexible electronics and screens. In addition, these silver-based films could enable flexible solar cells to be installed on roofs, windows, as well as personal devices.
The researchers have reported the development of a transparent conducting thin-film on glass discs measuring 10 centimeters in diameter in the journal, Optical Materials Express. On the basis of hypothetical estimations that closely matched with experimental measurements, they estimate that the thin-film electrodes could perform considerably better than those employed for present touch screens and flexible displays.
The approach we used for fabrication is highly reproducible and creates a chemically stable configuration with a tunable tradeoff between transparency and conductive properties. This means that if a device needs higher transparency but less conductivity, the film can be made to accommodate by changing the thickness of the film.
Jes Linnet, First Author - University of Southern Denmark
Finding a Flexible Alternative
Indium tin oxide (ITO) can exhibit a transparency of up to 92% - similar to glass, and majority of present transparent electrodes are made of this material. While ITO thin films are highly transparent, they also come with certain drawbacks - for example, they are too brittle to use with flexible displays or electronics and have to be carefully processed to achieve reproducible performance. Due to these drawbacks, scientists are looking for alternatives to ITO.
Noble metals such as silver, gold, and platinum have anti-corrosive properties, which make them as promising alternatives to ITO for making chemically-resistant, long-lasting electrodes that could be employed with flexible substrates. Yet, until now, high surface roughness has been a characteristic of noble metal transparent conductive films that can degrade performance. This is because the interface between the film and other layers is not flat. Although carbon nanotubes can be used for making transparent conductive films, these films do not presently have sufficient conductance for all applications and are likely to have surface roughness because the nanotubes are stacked on top of one another.
In the latest study, the researchers developed transparent conductive silver thin films by applying a new method known as colloidal lithography. To achieve this, they initially developed a masking template, or layer, by coating a layer of evenly sized, close-packed plastic nanoparticles to a 10-centimeter wafer. The researchers then reduced the size of all the particles uniformly by placing the coated wafers into a plasma oven. When a thin silver film was deposited onto the masking layer, the silver was able to penetrate the spaces between the particles. Next, the team dissolved the particles, leaving an accurate pattern of honeycomb-like holes that enable the passage of light and eventually create an optically transparent and electrically conductive film.
Balancing Transparency and Conductivity
The scientists demonstrated that by applying their large-scale fabrication technique, silver transparent electrodes with as much as 80% transmittance can be created, while electrical sheet resistance can be kept below 10 ohms per square – roughly a tenth of what has been reported for films based on carbon-nanotube with the similar transparency. If the electrical resistance is lower, the electrodes will conduct an electrical charge in a better way.
The most novel aspect of our work is that we accounted for both the transmission properties and the conductance properties of this thin film using theoretical analysis that correlated well with measured results. Fabrication problems typically make it hard to get the best theoretical performance from a new material. We decided to report what we encountered experimentally and postulate remedies so that this information could be used in the future to avoid or minimize problems that may affect performance.
Jes Linnet, First Author - University of Southern Denmark
According to the research team, the findings demonstrate that colloidal lithography can be used for creating chemically stable transparent conductive thin films and could also prove useful for a wide range of applications.