Jul 9 2018
A team of Chinese researchers reported that it had achieved success in nitrogen metallization at extreme conditions. This breakthrough result has been reported in Nature Communications on July 6.
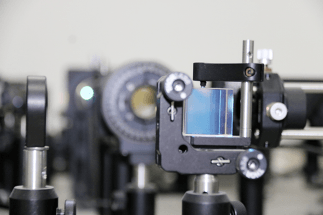 The team developed its own pulsed-laser heating system and ultra-fast optical detection technology (Image by YAO Jie)
The team developed its own pulsed-laser heating system and ultra-fast optical detection technology (Image by YAO Jie)
Working at the Institute of Solid State Physics at the Hefei Institutes of Physical Science (CASHIPS), the researchers developed their own ultra-fast optical detection technology and pulsed-laser heating system to perform the experiment.
Nitrogen represents approximately 78% of air by volume. It usually maintains in a fairly stable molecular gas state without any odor or color. However, nitrogen was anticipated to present relatively different properties with exposure to adverse conditions. For example, nitrogen is believed to convert into a metal in the Earth’s core, and is regarded as one of the most energetic materials to exist in our planet.
For many years, researchers have been trying to synthesize a range of materials with very high energy based on nitrogen, particularly metallic and polymeric nitrogen. In addition, it is particularly relevant to investigate metallic nitrogen at very high temperatures and pressures considering the difficulties of consistent achievement of metallic hydrogen—another equally important material that is expected to be a room-temperature superconductor.
Actually, a number of attempts were made using many different techniques in earlier studies, for example, dynamic pressure research with a gas gun and static pressure studies with diamond anvil cell.
The earlier results provided some specific understanding about the “hologram” of pressure-temperature-state corresponding data of nitrogen, but this insight was rather limited.
The key is to understand the whole pressure-temperature conditions concerning the transition from molecular nitrogen to metallic nitrogen.
Alexander Goncharov, a member of China’s "Thousand Talent Program," who heads a study team.
Goncharov also observed that a specific difficulty in this kind of analysis is the mechanism of transition from insulator to metal, and the location and existence of the vital point where the transition character alters.
JIANG Shuqing, the paper’s first author and also a scientist working with CASHIPS, informed that the researchers used their self-developed experimental system to scan the hologram of the nitrogen properties at a fairly wide range of extreme conditions—from 0 to 170 GPa and up to approximately 8000 K.
This allowed us to determine the pressure-temperature region of metallic nitrogen. It’s generally above 125 GPa and 2500 K. The result provided us detailed information on insulator-to-metal transition in nitrogen.
JIANG Shuqing
JIANG concluded that the researchers believe that these observations provide a better insight into the interplay between melting, molecular dissociation, and metallization, thereby exposing features that are also typical in hydrogen.
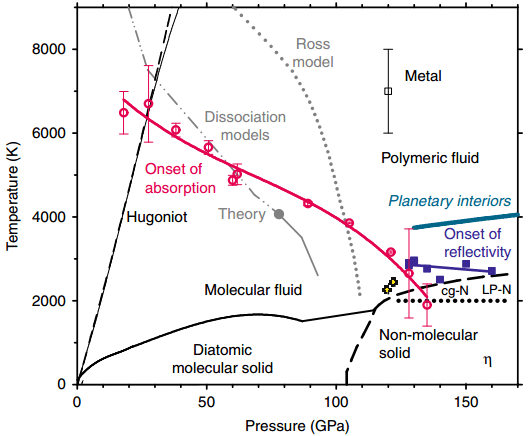
The P-T phase diagram of nitrogen (Image by JIANG Shuqing)