Sep 3 2018
The natural substance 3-carene is a constituent of turpentine oil, a waste stream of the manufacture of cellulose from wood. Until now, this by-product has been mostly burnt. Fraunhofer scientists are employing new catalytic processes to change 3-carene into building blocks to make bio-based plastics. The new polyamides are transparent as well as possess a high thermal stability.
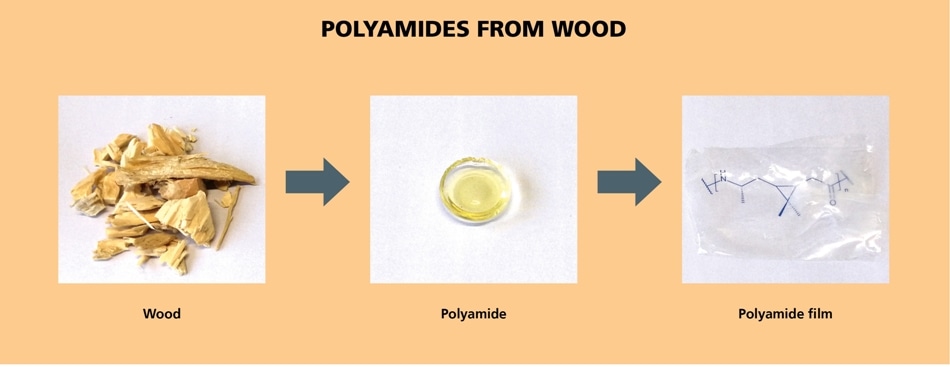 From wood waste to high-performance polymers: Terpenes from turpentine are converted to bio-based, transparent and heat-stable polyamides under application of a new catalytic process. (© Fraunhofer IGB)
From wood waste to high-performance polymers: Terpenes from turpentine are converted to bio-based, transparent and heat-stable polyamides under application of a new catalytic process. (© Fraunhofer IGB)
Plastics are a beneficial alternative to metal or glass for a wide variety of applications. Polyamides play a vital role in the production of superior-quality structural components, as they are not only impact- and abrasion-resistant, but also resistant to a number of solvents and chemicals. Nowadays, polyamides are chiefly produced from crude oil.
A sustainable alternative: monomers from wood waste
The Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB is exploring a sustainable substitute for the manufacture of new high-performance plastics from terpenes found in resin-rich wood. The natural substances can be gotten from conifers such as larch, pine, or spruce. In the making of pulp, in which wood is broken down to separate the cellulose fibers, the terpenes are isolated in huge quantities as a by-product, turpentine oil.
In the collaborative project “TerPa–Terpenes as building blocks for bio-based polyamides”, scientists at the Straubing BioCat branch of Fraunhofer IGB have currently succeeded in enhancing the synthesis of lactams from the terpene 3-carene and changing them into a scalable, competitive process on a possibly industrial scale. Lactams are building blocks for the creation of polyamides. The Straubing experts could already demonstrate that terpenes such as limonene, α-pinene, and 3-carene are appropriate raw materials for the synthesis of bio-based lactams.
Economical one-pot reaction sequence
The transformation of 3-carene to the corresponding lactam requires four consecutive chemical steps. The special characteristic of the patent-pending Straubing solution is that the conversions can occur as a “one-pot reaction sequence” in a single reactor—purification of the intermediate products is not necessary. “We have achieved this by carefully selecting the catalysts and reaction conditions—and it saves time and money,” Paul Stockmann explains, who developed and enhanced the promising process.
“Even on a laboratory scale, our process delivers more than 100 grams of diastereomerically pure lactam monomer per production run. This quantity is quite sufficient for initial investigations of the production and evaluation of the new plastics,” Stockmann said. Another benefit: No toxic or environmentally dangerous chemicals are needed for the synthesis of the lactam.
Bio-based, transparent, thermally stable
However, that is not all. Owing to the unusual chemical structure of 3-carene, the side chains of the natural compound constrain the crystallization of the resulting polymer. “Our bio-based polymers are therefore predominantly ‘amorphous’ and thus transparent which is very unusual for bio-based polyamides,” says Dr. Harald Strittmatter, who heads the project at the BioCat branch in Straubing. This makes the new polyamides ideal as protective shields, for instance in ski goggles or visors. They can also be made with significantly less energy input than petroleum-based transparent polyamides. In contrast to other bioplastics, which are mostly made from wheat, corn, or potato starch, bio-based polyamides do not compete with food production. Rather, they bring value to a waste stream that, up to now, has been burned for energy production.
Another benefit: The new bio-based polyamides also have exceptional thermal properties. “The glass transition point of our polyamides is 110 °C. They can therefore also be at permanently high temperatures, for example as components in the engine compartment of motor vehicles,” Strittmatter says. It is true that polyamides created from fossil resources possess similar temperature properties. But, because of their aromatic domains - which do not occur in the 3-carene based polyamides - they discolor over time under the impact of UV light, restricting their potential for outdoor applications.
Carenlactams give PA12 and PA6 new properties
The researchers have also polymerized the bio-based lactams with other commercially available monomer molecules - laurolactam (monomer of PA12) and caprolactam (monomer of PA6) - to form copolymers. The crystallinity and therefore the transparency of the new copolymers were considerably altered. In principle, the application profiles of the extensively used plastics PA6 and PA12 are potentially extended.
Following additional enhancement of monomer synthesis, colleagues at the Fraunhofer Institute for Environmental, Safety and Energy Technology UMSICHT in Oberhausen will transfer the process to the 20-liter pilot scale and make larger sample quantities of lactams. The properties of the new polymers and copolymers will then be examined in more detail to identify probable applications. The researchers also plan to explore the biodegradability of the new polyamide. The Fraunhofer scientists hope that interested companies will then be able to transform the results to industrial scale.