Nov 27 2018
A new category of carbides, anticipated to be one of the hardest materials with the highest melting points that exist on Earth, has been discovered by materials scientists at Duke University and UC San Diego. Developed using low-cost metals, the innovative materials may soon find applications in a broad array of industries from hardware and machinery to aerospace.
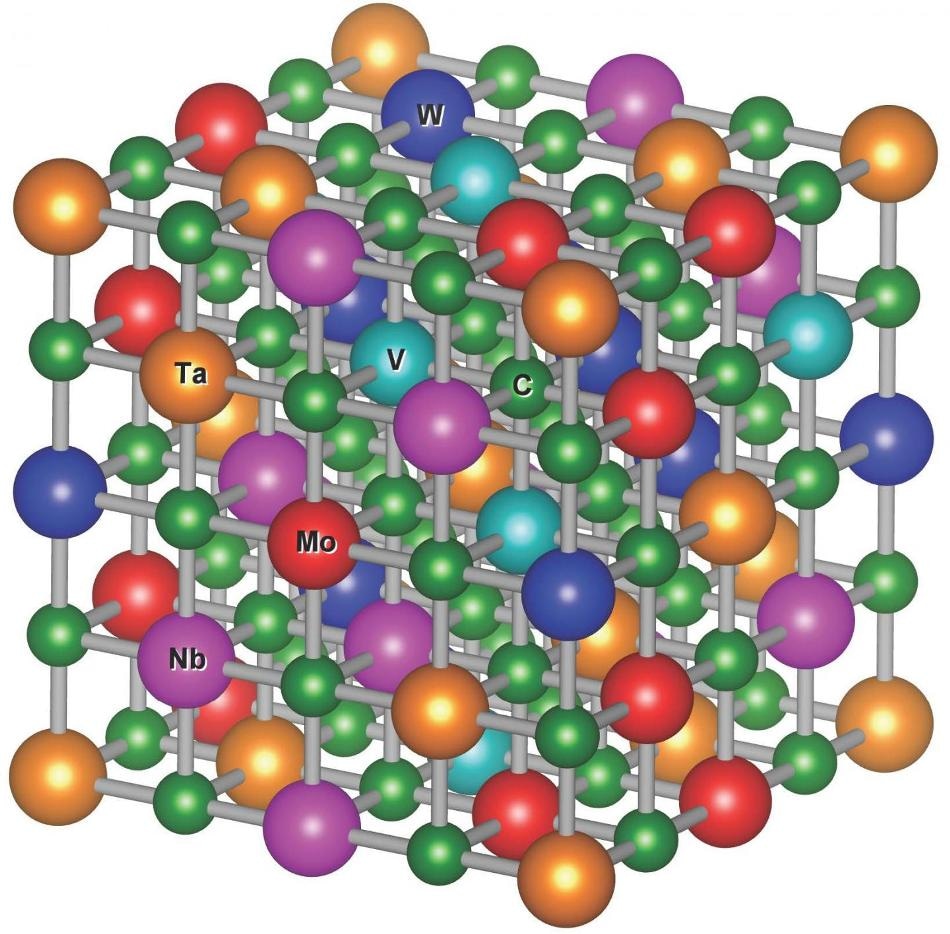 A computer model of the atomic structure of one of the new carbides. The jumbled mess of carbon and five metal elements gives stability to the overall structure. (Image credit: Pranab Sarker, Duke University)
A computer model of the atomic structure of one of the new carbides. The jumbled mess of carbon and five metal elements gives stability to the overall structure. (Image credit: Pranab Sarker, Duke University)
Conventionally, a carbide is a compound made of carbon and one other element. When combined with a metal like tungsten or titanium, the ensuing material is exceptionally hard and hard to melt. This renders carbides perfect for applications such as coating the parts of a space vehicle or surface of cutting tools.
Although there exist a small number of complex carbides that contain three or more elements, they are not usually found outside the laboratory or in industrial applications. This is mainly because of the challenges in determining the combinations that can form stable structures, not to mention desirable properties.
At present, a group of materials scientists from Duke University and UC San Diego has reported the discovery of a new category of carbides that combine carbon with five distinctive metallic elements at the same time. The outcomes of the study have been published online in the Nature Communications journal on November 26th, 2018.
Realizing stability from the disordered mixture of their atoms as opposed to orderly atomic structure, these materials were computationally predicted to exist by the Duke University scientists and then successfully produced at UC San Diego.
These materials are harder and lighter in weight than current carbides. They also have very high melting points and are made out of relatively cheap material mixtures. This combination of attributes should make them very useful to a wide range of industries.
Stefano Curtarolo, Professor of Mechanical Engineering and Materials Science, Duke University.
While learning about molecular structures, students are shown crystals like salt, which looks like a 3D checkerboard. The strength and stability of these materials arise from their regular, ordered atomic bonds where the atoms fit together similar to pieces of a jigsaw puzzle.
However, flaws in a crystalline structure can usually increase the strength of a material. If, for instance, cracks start spreading along a line of molecular bonds, a group of misaligned structures can stop it in its tracks. The process of hardening solid metals by producing the ideal amount of disorder is realized through a process of heating and quenching known as annealing.
The new category of five-metal carbides takes this concept to the next level. Eliminating any dependence on crystalline structures and bonds for their stability, these materials are solely dependent on the disorder. Although it is not possible for a pile of baseballs to stand on its own, a pile of baseballs, bats, shoes, gloves, and hats just might.
The challenge lies in predicting the combination of elements that will stand firm. Attempting to develop innovative materials is time-consuming and expensive. It is more expensive to compute atomic interactions through first principle simulations. Moreover, with five slots for metallic elements and 91 to choose from, the number of prospective recipes quickly turns horrifying.
“To figure out which combinations will mix well, you have to do a spectral analysis based on entropy,” stated Pranab Sarker, a postdoctoral associate in Curtarolo’s lab and one of the first authors of the paper. “Entropy is incredibly time-consuming and difficult to calculate by building a model atom-by-atom. So we tried something different.”
First, the researchers narrowed the field of ingredients to eight familiar metals known to produce carbide compounds with high melting temperatures and hardness. Subsequently, they calculated the amount of energy needed for a potential five-metal carbide to form a large set of random configurations.
If the outcomes were spread far apart, it suggested that the combination would probably favor a single configuration and break apart—analogous to having too many baseballs in the combination. However, if there were several configurations tightly bundled together, it suggested the material would possibly form various distinctive structures all at the same time, thereby offering the disorder required for structural stability.
Then, the researchers tested their hypothesis by getting their teammate Kenneth Vecchio, professor of NanoEngineering at UC San Diego, to try to actually produce nine of the compounds. This was performed by integrating the elements in each recipe in a finely powdered form, compressing them at temperatures up to 4000 °F and running 2000 A of current directly through them.
“Learning to process these materials was a difficult task,” stated Tyler Harrington, a PhD student in Vecchio’s lab and co-first author of the paper. “They behave differently than any materials that we’ve ever dealt with, even the traditional carbides.”
They selected the three recipes their system deemed most probable to form a stable material, the two least probable, and four random combinations that scored in between. According to the predictions, the three most probable candidates were successful, whereas the two least probable were not. Stable structures were also formed by three of the four intermediate scorers.
Although it seems probable that the new carbides all have desirable industrial properties, one unlikely combination came to the fore—a combination of molybdenum, niobium, tantalum, vanadium, and tungsten called MoNbTaVWC5 for short.
Getting this set of elements to combine is basically like trying to squeeze together a bunch of squares and hexagons. Going on intuition alone, you’d never think that combination would be feasible. But it turns out that the best candidates are actually counterintuitive.
Cormac Toher, Assistant Research Professor, Curtarolo’s Laboratory.
“We don’t know its exact properties yet because it hasn’t been fully tested,” stated Curtarolo. “But once we get it into the laboratory in the next couple of months, I wouldn’t be surprised if it turned out to be the hardest material with the highest melting point ever made.”
“This collaboration is a team of researchers focused on demonstrating the unique and potentially paradigm-changing implications of this new approach,” stated Vecchio. “We are using innovative approaches to first-principles modeling combined with state-of-the-art synthesis and characterization tools to provide the integrated ‘closed-loop’ methodology so necessary for advanced materials discovery.”
This study was supported by the Department of Defense Office of Naval Research (N00014-15-1-2863, N00014-17-1-2090, N00014-16-1-2583, N00014-17-1-2876).