Dec 11 2018
Batteries that outpace the present-day lithium-ion range with regard to how much energy they can pack into a small lightweight platform, could prod electric vehicle usage into high gear. A new research specifying an electrochemistry advance may stimulate one such high-energy-density type, the fluoride-ion battery (FIB), from the drawing board toward real-world usage.
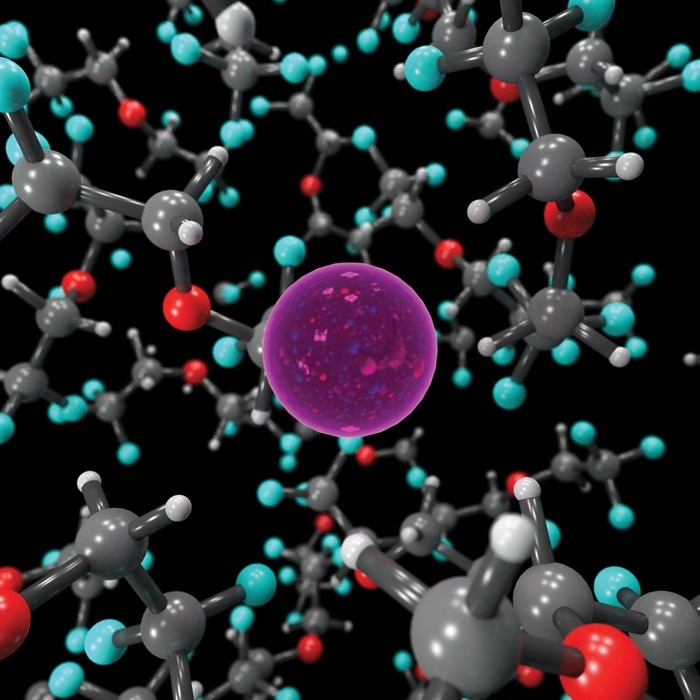 This image from a computer simulation depicts the molecular interactions between a fluoride ion (pink) and a flouroethylether electrolyte solvent. (Image credit: Brett M. Savoie/Caltech/Purdue)
This image from a computer simulation depicts the molecular interactions between a fluoride ion (pink) and a flouroethylether electrolyte solvent. (Image credit: Brett M. Savoie/Caltech/Purdue)
Rechargeable FIBs, which theoretically can contain about eight times as much energy per volume as existing lithium-ion batteries can, are not new, but they are rare. That is because these devices produce electricity by ferrying fluoride ions from one electrode to the other through a fluoride-ion-conducting electrolyte. The electrolytes are solids, and to persuade them to conduct ample ion currents, they have to be heated above 150 °C, which extremely limits applications.
Currently, a large team of scientists, including Simon C. Jones of California Institute of Technology and Christopher J. Brooks of the Honda Research Institute, have developed a liquid electrolyte that ferries fluoride ions to and fro and proved its use in a room-temperature, rechargeable FIB (Science 2018, DOI: 10.1126/science.aat7070).
To produce the electrolyte solution, the researchers hunted for a combination of a fluoride salt and solvent that offered high ionic conductivity, sufficient solubility, and electrochemical stability. The search led to a neopentyl alkylammonium fluoride and bis(2,2,2-trifluoroethyl)ether, or BTFE.
Then the team built a novel cathode comprising of a copper core and a lanthanum trifluoride shell. The shell stops copper dissolution and BTFE decomposition while permitting facile diffusion of fluoride ions between the liquid electrolyte and the copper core. The diffusion allowed reversible conversion of copper to copper fluoride during charging cycles. In a proof-of-concept showcase, the team created test cells using those components and worked them at room temperature for seven charging cycles.
“These results open up new opportunities for the scientific community” in high-energy-density batteries, says Jun Liu, a specialist in energy storage materials at Pacific Northwest National Laboratory. The research shows good progress, Liu notes, but it also highlights the need for “much more research in this area” to create a long-lasting, viable fluoride-ion battery that can surpass the performance of Li-ion batteries with regard to charge capacity.