Dec 14 2018
“Urban mining”—or the recycling of precious metals from used electronic gadgets—has turned out to be important than ever, despite the lack of processes that are both efficient and environmentally benign.
 © Wiley-VCH
© Wiley-VCH
Currently, an international research team has taken a deep look into gold dissolution, particularly how organic thiol-containing compounds help dissolve elemental gold. The fast, selective, and convenient thiol-assisted gold leaching processes have been proposed in their study published in the journal Angewandte Chemie.
Melting is the conventional method used for recycling gold “waste.” Dental gold and jewelry can be recycled about 100%; however, recycling of precious metals in computers, smartphones, and other electronic gadgets is much more difficult, and the recovery quote is even low. Although they are abundant in electronic devices, their relative content is still very low to enable really economical urban mining.
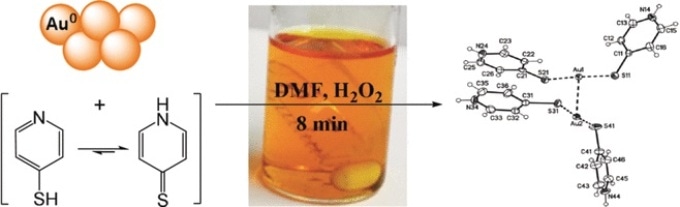
Hydrometallurgical cyanide leaching is the conventional mining method for gold and it produces a large amount of hazardous waste while being comparatively unselective. Many of the latest concepts are based on the complexation of gold in organic solutions as it forms soluble complexes with sulfur-containing reagents. However, the processes must be viable on a large scale and still prevent the formation of hazardous or toxic compounds. Currently, Timo Repo from the University of Helsinki, Finland, and his team have deeply examined the details of selective gold extraction in organic solution. They put forward an efficient gold recovery method from electronic waste using the chemical dimethyl formamide as organic solvent, pyridinethiols and hydrogen peroxide as reagents, and, optionally, elemental sulfur to minimize the reagent load.
Pyridinethiol is pyridine, a nitrogen-containing aromatic ring, with a thiol group, SH, attached to its ring. The reagent binds elemental gold to form soluble complexes, and in addition, the complex has a favorable linear structure created by two pyridinethiol molecules on either side of the gold atom. Upon oxidation, it changes to a stable cationic gold-containing product in organic solution. This complex formation with two ligands is a unique feature of gold, facilitating the energetics of dissolution and oxidation. As a result, the authors reported nearly quantitative dissolution of gold from film, powder, or electronic boards after the extraction time of 20 minutes.
But how can gold dissolution be differentiated from that of other precious metals? Unlike gold having a one-electron oxidation, palladium and platinum need two-electron oxidations and hence are not accessible with this technique. On the contrary, copper as well as silver form complexes with pyridinethiols, although not as effective as gold. Hence, prior to dissolving the gold from the “gold finger” region in a printed circuit board, the researchers first extracted silver and copper with ammonia and sulfate-containing solutions, which are traditional methods.
By analyzing the exact mechanism of thiol-assisted gold dissolution, the researchers found a surprisingly high variety of sulfur-containing side products. Some of them appeared to be critical for proceeding the oxidation reaction, such as S8, a common form of elemental sulfur. This also proved to be an asset: when an external S8 is added, it was possible to reduce the ligand load, according to the authors. Their extraction technique could mark a new beginning for more efficient urban mining.