Jan 11 2019
Lithium-air batteries are ready to become the next ground-breaking alternatives for existing lithium-ion batteries that power computers, cell phones, and electric vehicles.
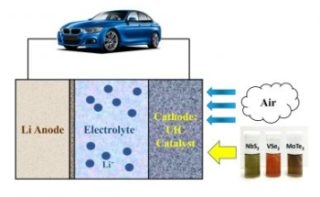 2D catalysts power an electric vehicle. (Image Credit: Amin Salehi-Khojin)
2D catalysts power an electric vehicle. (Image Credit: Amin Salehi-Khojin)
Lithium-air batteries, which are at present in the experimental stages of development, have the ability to store 10 times more energy compared to lithium-ion batteries, and they are considerably lighter in weight. Having said that, the integration of sophisticated catalysts made from two-dimensional (2D) materials could make lithium-air batteries even more efficient and offer more charge. Catalysts assist in increasing the rate of chemical reactions within the batteries, and based on the type of material the catalyst is produced from, they can considerably improve the ability of the battery to store and supply energy.
We are going to need very high-energy density batteries to power new advanced technologies that are incorporated into phones, laptops, and especially electric vehicles.
Amin Salehi-Khojin, Associate Professor, Mechanical and Industrial Engineering, UIC College of Engineering.
Many 2D materials that can serve as catalysts were produced by Salehi-Khojin and his team. Upon incorporating a number of their 2D materials as the catalyst into experimental lithium-air batteries, the battery was able to store up to 10 times more energy when compared to lithium-air batteries consisting of conventional catalysts. The results of the study have been reported in the Advanced Materials journal.
“Currently, electric vehicles average about 100 miles per charge, but with the incorporation of 2D catalysts into lithium-air batteries, we could provide closer to 400 to 500 miles per charge, which would be a real game-changer,” stated Salehi-Khojin, who is also the corresponding author of the paper. “This would be a huge breakthrough in energy storage.”
Fifteen different types of 2D transition metal dichalcogenides (TMDCs) were produced by Salehi-Khojin and his teammates. TMDCs are considered as unique compounds due to their high electronic conductivity and fast electron transfer that can be used to take part in reactions with other materials, for example, the reactions that occur inside batteries during charging and discharging.
The researchers experimentally analyzed the performance of 15 TMDCs as catalysts in an electrochemical system that mimics a lithium-air battery.
In their 2D form, these TMDCs have much better electronic properties and greater reactive surface area to participate in electrochemical reactions within a battery while their structure remains stable. Reaction rates are much higher with these materials compared to conventional catalysts used such as gold or platinum.
Leily Majidi, Graduate Student, UIC College of Engineering.
Majidi is the first author of the study. One of the reasons behind the optimal performance of the 2D TDMCs is that they help accelerate charging as well as discharging reactions taking place in lithium-air batteries.
“This would be what is known as bi-functionality of the catalyst,” Salehi-Khojin said.
Furthermore, the 2D materials synergize with the electrolyte, the material through which ions move at the time of the charge and discharge cycles.
The 2D TDMCs and the ionic liquid electrolyte that we used acts as a co-catalyst system that helps the electrons transfer faster, leading to faster charges and more efficient storage and discharge of energy. These new materials represent a new avenue that can take batteries to the next level, we just need to develop ways to produce and tune them more efficiently and in larger scales.
Amin Salehi-Khojin, Associate Professor, Mechanical and Industrial Engineering, UIC College of Engineering.
The co-authors of the paper include Poya Yasaei, Zahra Hemmat, Pedram Abbasi, Shadi Fuladi, Xuan Hu, Robert Klie, Fatemeh Khalili-Araghi, and Baharak Sayahpour of the University of Illinois at Chicago and Robert Warburton and Jeffrey Greeley of Purdue University.
The study was supported in part by the National Science Foundation DMREF Grant 1729420.