Mar 4 2019
Supported single metal atoms have gained wide attention due to their established high efficiency in single metal catalysis. However, the preparation of such catalysts remains difficult since the neutral metal atoms strongly tend to agglomerate to metal particles in characteristic preparations.
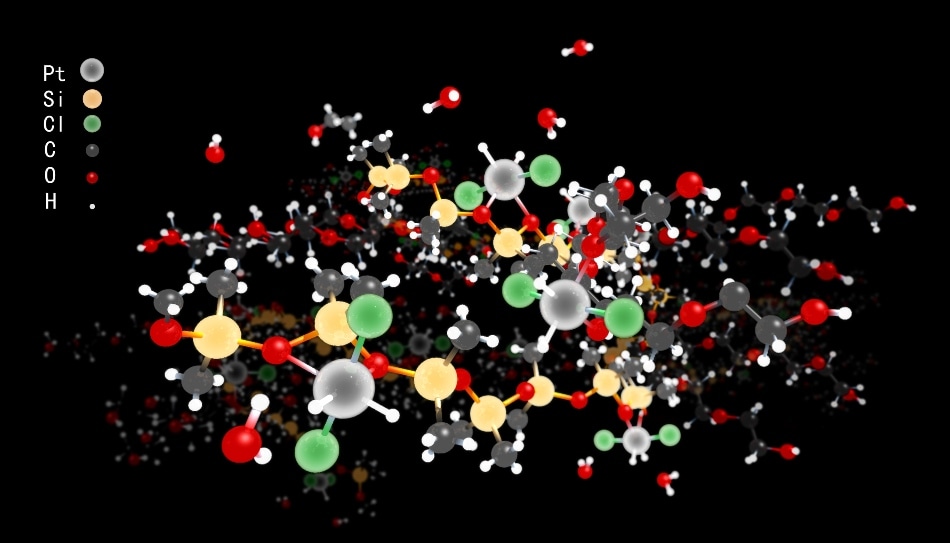 Schematic illustration of (R1OR2)2Pt(0)Cl2H2. (Image Credit: DICP)
Schematic illustration of (R1OR2)2Pt(0)Cl2H2. (Image Credit: DICP)
Scientists at the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences and the University of Delaware have described a method to make a colorless liquid reservoir of metal-like discrete platinum atoms. The study outcomes have been reported in Nature Communications.
Alcohols reduce platinum chloride salts to single platinum metal atoms in an environmentally gentle liquid surfactant. A mantle of hydrochlorides protects the individual Pt atoms, which are docked in the liquid by abundant oxygen atoms. It is possible to scale the preparation of the metal-like Pt atoms.
Since platinum is a noble metal, metallic Pt nanoparticles on carbon or oxide supports are extensively employed in petroleum refining and chemical industries owing to their distinctive catalytic functions.
The reserve of Pt on earth is limited, and about 5.6 tons of Pt are consumed every year just in the silicone industry.
Z. Conrad Zhang, Lead Researcher, Dalian Institute of Chemical Physics, Chinese Academy of Sciences.
The catalytic performance of the liquid loaded with Pt atoms was tested by the scientists.
We found that the electron-deficient Pt atoms in the liquid exhibited super-high activity and high selectivity for the reaction compared to known Pt catalysts.
LIU Kairui, Study Lead Author and Graduate Student, Chinese Academy of Sciences.
Since the docked discrete Pt atoms do not get aggregated under reaction conditions, they retain high activity and remain colorless through constant applications.
“The high activity, selectivity, and stability of this catalyst may dramatically reduce the amount of Pt consumed by the silicone industry and may be broadly applicable to other applications,” stated Zhang.
The liquid loaded with the Pt atoms is stable at 120 °C and stays clear for more than six months on the shelf at ambient conditions, yet it was found by the scientists that it became dark as a result of aggregation of the Pt atoms upon being exposed to X-ray or electron beams usually used to characterize the Pt atoms. To solve this issue, the scientists used 195Pt nuclear magnetic resonance (NMR) spectroscopy as the tool, which was found to offer explicit evidence for the formed Pt atoms.
The NMR spectroscopic data of the liquid not only unambiguously showed the discrete nature of mononuclear Pt atoms but also revealed only one carbon monoxide coordinated to a Pt atom.
BAI Shi, Professor, University of Delaware.
We are expanding the depositories of various metal atoms in our current research. The successful synthesis of readily removable mantles of the Pt atoms in the liquid phase may potentially enable atomically controllable fabrication of catalytic materials and metallic materials by design.
Z. Conrad Zhang, Lead Researcher, Dalian Institute of Chemical Physics, Chinese Academy of Sciences.