Apr 4 2019
Ketones are vital organic compounds found in a range of agrochemicals, pharmaceuticals, and natural products. The direct synthesis of a ketone from an equally readily-available aldehyde would be suitable and has been effectively carried out using a metal catalyst. However, metal catalysts are costly and the metal remaining in the end products may cause complications.
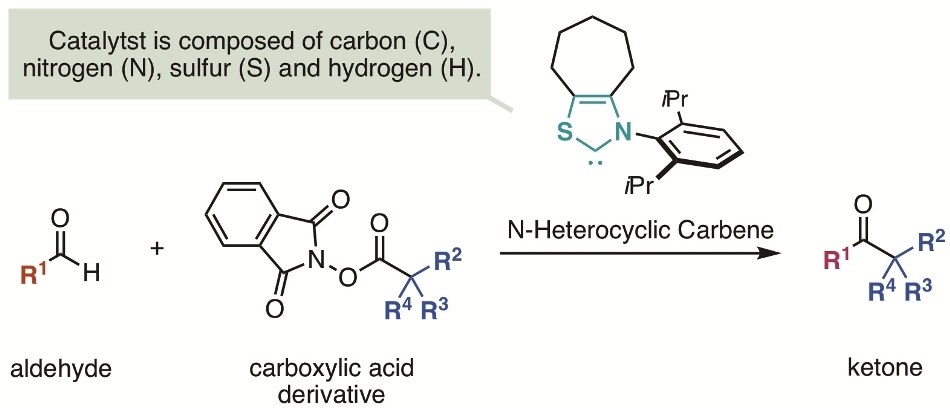 Outline of research. (Image credit: Kanazawa University)
Outline of research. (Image credit: Kanazawa University)
On the other hand, N-heterocyclic carbine, an organic compound, is said to serve as an organic catalyst and for such synthesis. N-heterocyclic carbene reacts with an aldehyde to develop a Breslow intermediate. This Breslow intermediate, serving as a nucleophile followed by two-electron transfer, reacts with different electrophiles to produce a ketone. However, this carbon–carbon bond formation after two-electron transfer is said to be sterically stalled and N-heterocyclic carbene is identified to react only with electrophiles with activated electrons.
Outline of research results
The team at Kanazawa University, including two students, has been successful in synthesizing a ketone from a carboxylic acid and an aldehyde by using N-heterocyclic carbene catalyst.
The main reason for the success of the reaction formulated in this research was the discovery of a new reaction process in which radical–radical coupling followed by carbon–carbon bond formation occurred after one electron transfer happened from a Breslow intermediate of an enolate produced from an aldehyde and an N-heterocyclic carbene to an electrophile. Since an extremely reactive radical is employed in the process of the carbon–carbon bond formation, it is viable to add a sterically bulky alkyl substituent, which used to be tough in such a reaction.
Furthermore, the team was successful in transforming a carboxylic acid, part of a number of pharmaceutical drugs and natural products, into a ketone by the technique illustrated here.
Future prospects
The team at Kanazawa University was successful in synthesizing a ketone, a crucial backbone of several pharmaceuticals, from a readily available aldehyde and a carboxylic acid. The technique makes conceivable the fast synthesis of ketones even with bulky substituents, as well as those derived from natural products or pharmaceuticals.
Accordingly, their technique is anticipated to speed-up drug discovery.
From a truly scientific perspective, it should be kept in mind that the new reaction process, i.e. a radical–radical coupling after one electron transfer arising from a Breslow intermediate, will be a new design parameter for N-heterocyclic carbene catalytic reactions.