Apr 10 2019
Electric vehicles are powered by lithium batteries, enabling them to travel many hundred miles on a single charge. While it is known that these batteries have the capacity for storing energy, they also have a tendency to catch fire on an occasional basis. Battery researchers call this occurrence “thermal runaway.”
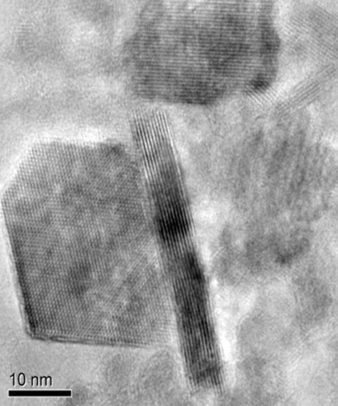 Lithium cobalt oxide particles coated in graphene. (Image credit: Reza Shahbazian-Yassar.)
Lithium cobalt oxide particles coated in graphene. (Image credit: Reza Shahbazian-Yassar.)
Most often, these fires happen when the batteries cycle or overheat quickly. Every year, an increasing number of electric vehicles are introduced on the road, and as a result, battery technology has to adjust to reduce the chances of these fires which can be both dangerous and catastrophic.
Now, at the University of Illinois at Chicago College of Engineering, (UIC College of Engineering), researchers have reported that the wonder material of the 21st century—graphene—may help in removing the oxygen from lithium battery fires. They have reported the results of their study in the journal Advanced Functional Materials.
High temperatures in the battery, and rapid charging and discharging or cycling cause lithium batteries to catch fire. Conditions like these can cause the cathode within the battery to decompose and discharge oxygen. In the case of a majority of lithium batteries, a lithium-containing oxide, typically lithium cobalt oxide, is used as the cathode.
Spontaneous combustion can take place when the oxygen mixes with other flammable products produced through electrolyte decomposition under sufficiently high heat.
We thought that if there was a way to prevent the oxygen from leaving the cathode and mixing with other flammable products in the battery, we could reduce the chances of a fire occurring.
Reza Shahbazian-Yassar, Study Corresponding Author and Associate Professor, Department of Mechanical and Industrial Engineering, UIC College of Engineering
Eventually, a material which is quite familiar to Shahbazian-Yassar offered an ideal solution to this challenge. That material happened to be graphene, an extremely thin layer of carbon atoms with exceptional characteristics. Earlier, graphene was used by Shahbazian-Yassar and his colleagues to help modify the buildup of lithium on electrodes present in lithium metal batteries.
The team was aware that graphene sheets are impervious to oxygen atoms. Moreover, graphene is flexible, robust, and can be designed to be electrically conductive.
Soroosh Sharifi-Asl, lead author of the paper and a graduate student in mechanical and industrial engineering at UIC, and Shahbazian-Yassar believed that if extremely small particles of the lithium cobalt oxide cathode of a lithium battery are wrapped in graphene, oxygen can be prevented from escaping.
Initially, the scientists made the graphene electrically conductive by chemically modifying it and then filled the conductive graphene with the extremely small particles of lithium cobalt oxide cathode electrode. With the help of electron microscopy, they viewed the graphene-wrapped lithium cobalt oxide particles and observed that the discharge of oxygen under extreme heat was considerably reduced when compared to unwrapped lithium cobalt oxide particles.
Afterward, the researchers used a binding material to bind the wrapped particles together to create a usable cathode and integrated it into a lithium metal battery. When the released oxygen was measured at the time of battery cycling, it was observed that practically no oxygen was escaping from cathodes even at extremely high voltages. Moreover, even after 200 cycles, the lithium metal battery went on to perform well.
The wrapped cathode battery lost only about 14% of its capacity after rapid cycling compared to a conventional lithium metal battery where performance was down about 45% under the same conditions.
Soroosh Sharifi-Asl, Study Lead Author and Graduate Student, Department of Mechanical and Industrial Engineering, UIC
Graphene is the ideal material for blocking the release of oxygen into the electrolyte. It is impermeable to oxygen, electrically conductive, flexible, and is strong enough to withstand conditions within the battery. It is only a few nanometers thick so there would be no extra mass added to the battery. Our research shows that its use in the cathode can reliably reduce the release of oxygen and could be one way that the risk for fire in these batteries—which power everything from our phones to our cars—could be significantly reduced.
Reza Shahbazian-Yassar, Study Corresponding Author and Associate Professor, Department of Mechanical and Industrial Engineering, UIC College of Engineering
Co-authors of the study are Soroosh Sharifi-Asl, Tara Foroozan, Mohammad Asadi, Yifei Yuan, Ramasubramonian Deivanayagam, Amin Salehi-Khojin, Boao Song, and Ramin Rojaee of the UIC College of Engineering; Perla Balbuena and Fernando Soto of Texas A & M University; and Xuanxuan Bi, Jun Lu, and Khalil Amine of Argonne National Laboratory.
The study was partly supported by the National Science Foundation award CMMI-1619743.