Apr 11 2019
The leading power source for electric vehicles and portable electronics is lithium-ion batteries (LIBs).
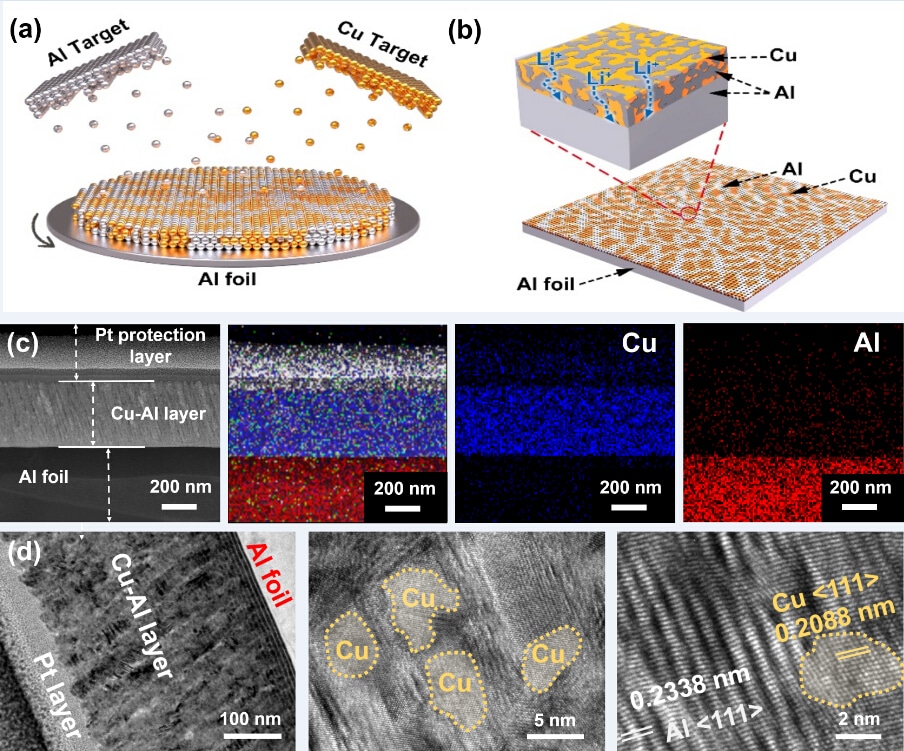 (a) Fabrication process for the inactive (Cu)/active (Al) layer by simultaneous deposition. (b) The 3D structure of Cu-Al@Al electrode. (c) Cross-section SEM images of the Cu-Al@Al and EDS element mapping. (d) Low- and high-resolution TEM of Cu-Al nanocomposite layer. (Image credit: TANG Yongbing)
(a) Fabrication process for the inactive (Cu)/active (Al) layer by simultaneous deposition. (b) The 3D structure of Cu-Al@Al electrode. (c) Cross-section SEM images of the Cu-Al@Al and EDS element mapping. (d) Low- and high-resolution TEM of Cu-Al nanocomposite layer. (Image credit: TANG Yongbing)
However, the comparatively low theoretical capacity of graphite anode (372 mAh g−1) hampers the improvement of the energy density of LIBs. Hence, leveraging anode materials with high capacity is gaining more and more attention.
Among the range of anode materials, aluminum (Al) is a potential candidate because of its natural abundance, low discharge potential, high theoretical capacity, outstanding conductivity, and particularly low cost. However, anodes based on aluminum are generally examined in half cells or full cells with low cathode areal density (<2 mg cm−2), which is beyond a practical requirement.
Recently, a team of scientists headed by Prof. TANG Yongbing and Dr ZHANG Miao at the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences published a paper titled “Uniform Distribution of Alloying/Dealloying Stress for High Structural Stability of Al Anode in High Areal Density Lithium Ion Battery” on Advanced Materials, which demonstrated how the scientists enhanced the cycling performance of Al-based batteries with a high areal density cathode.
In earlier studies, the group created a new LIB configuration with low cost and high efficiency, which used an integrated design of aluminum foil as an alternative to the graphite anode and the Cu current collector of traditional LIBs, leaving out traditional anode materials. Therefore, dead volume and dead weight could be significantly minimized, further increasing energy densities of this battery. However, this integrated anode also has an issue with cycling stability when connected with a high areal density cathode.
In this research, the researchers identified that the cracking and pulverization of the Al anode could be related to the irregular charge/discharge reaction along the boundaries of pristine Al, which resulted in the stress concentration and eventual failure of the Al anode. Later, they discovered that the lifetime of the Al anode can be prolonged through even distribution of the alloying/dealloying stress.
TANG and his partners supported an active (Al) and inactive (Cu) codeposition approach to uniformly distribute the alloying location and disperse the stress of volume expansion, which is advantageous in achieving the structural stability of the Al anode (called Cu-Al@Al).
The homogeneous reaction and uniform distribution of stress during the charge/discharge process enabled the full battery of Cu-Al@Al assembled with a high LiFePO4 cathode areal density of 7.4 mg cm−2 to obtain a capacity retention of approximately 88% over 200 cycles, which is the best performance of Al anodes in full batteries with a cathode of such high areal density.
The research implies that this inactive/active design offers a feasible method to solve the issue of Al anodes and provides opportunities for practical applications of Al anodes.