May 27 2019
A method that adds carbon-hydrogen molecules into a single atomic layer of the semiconducting material tungsten disulfide considerably alters the electronic properties of the material, according to Penn State scientists who say they can develop new types of components for energy-efficient photoelectric systems and electronic circuits using this material.
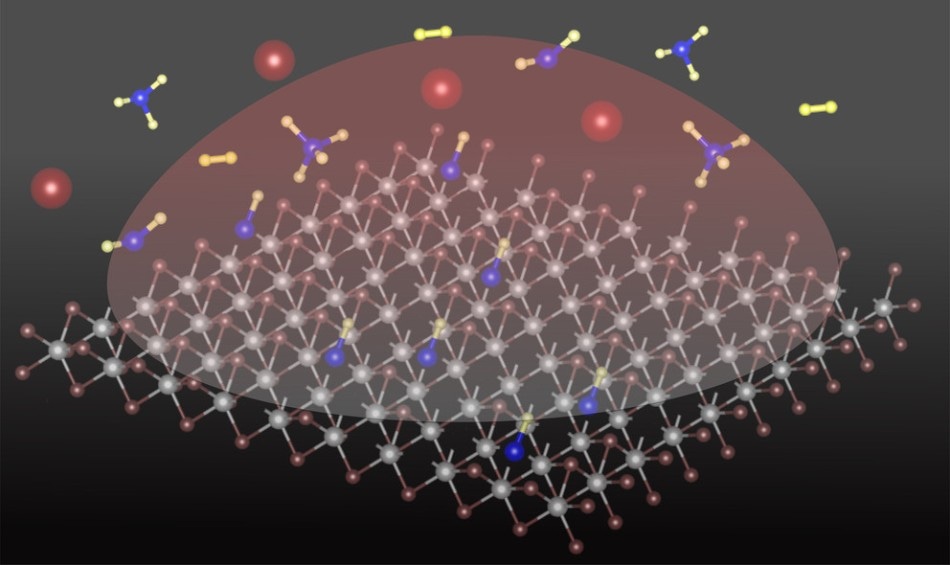 Schematic of plasma-assisted carbon-hydrogen species doping in the WS2 lattice. (Image credit: Fu Zhang, Penn State)
Schematic of plasma-assisted carbon-hydrogen species doping in the WS2 lattice. (Image credit: Fu Zhang, Penn State)
“We have successfully introduced the carbon species into the monolayer of the semiconducting material,” said Fu Zhang, doctoral student in materials science and engineering and lead author of a paper published online on May 26th in Science Advances.
Before doping — incorporating carbon — the semiconductor, a transition metal dichalcogenide (TMD), was n-type — electron conducting. After replacing carbon atoms for sulfur atoms, the one-atom-thick material formed a bipolar effect, a p-type — hole — branch, and an n-type branch. This gave rise to an ambipolar semiconductor.
“The fact that you can change the properties dramatically by adding as little as two atomic percent was something unexpected,” Mauricio Terrones, senior author and distinguished professor of physics, chemistry and materials science and engineering.
According to Zhang, after the material is extremely doped with carbon, the researchers can create a degenerate p-type with excellent carrier mobility. "We can build n+/p/n+ and p+/n/p+ junctions with properties that have not been seen with this type of semiconductor," he said.
With regards to applications, semiconductors are used in different devices in industry. Here, the majority of those devices will be transistors of various types. There are about 100 trillion transistors in a laptop.
This type of material might also be good for electrochemical catalysis. You could improve conductivity of the semiconductor and have catalytic activity at the same time.
Mauricio Terrones, Senior Author and Distinguished Professor of Physics, Chemistry and Materials Science and Engineering, Penn State University
There are some papers in the field of 2D materials doping, because it requires many processes to take place concurrently under specific types of settings. The team's method applied a plasma to bring down the temperature at which methane can be cracked — split apart — down to 752 °F. Simultaneously, the plasma has to be robust enough to push a sulfur atom out of the atomic layer and replace a carbon-hydrogen unit.
It’s not easy to dope monolayers, and then to measure carrier transport is not trivial. There is a sweet spot where we are working. Many other things are required.
Mauricio Terrones, Senior Author and Distinguished Professor of Physics, Chemistry and Materials Science and Engineering, Penn State University
Susan Sinnott, professor and head of the Department of Materials Science and Engineering, delivered the theoretical calculations that directed the experimental efforts. When Terrones and Zhang noticed that doping the 2D material was altering its electronic and optical properties —something they had never observed previously — Sinnott's team predicted the ideal atom to dope with and predicted the properties, which matched with the experiment.
Saptarshi Das, assistant professor of engineering science and mechanics, and his group, then measured the carrier transport in different transistors with increasing quantities of carbon substitution. They observed the conductance transform drastically until they had totally changed the conduction type from negative to positive.
“It was very much a multidisciplinary work,” Terrones says.
Additional authors on the Science Advances paper, titled “Carbon doping of WS2 monolayers: Bandgap reduction and p-type doping transport,” include current or former doctoral students Yanfu Lu, Daniel Schulman, Tianyi Zhang, Zhong Lin, and Yu Lei; and Ana Laura Ellias and Kazunori Fujisawa, assistant research professors of physics.
This work was supported by the Basic Energy Sciences program in the Department of Energy’s Office of Science.