Jul 9 2019
The electrical characteristics of dihexyl-quarterthiophene (DH4T) thin films typically rely on their structure. Now, a research team from France, Germany, and Russia, involving materials scientists from the Moscow Institute of Science and Technology, has examined the reason behind this phenomenon.
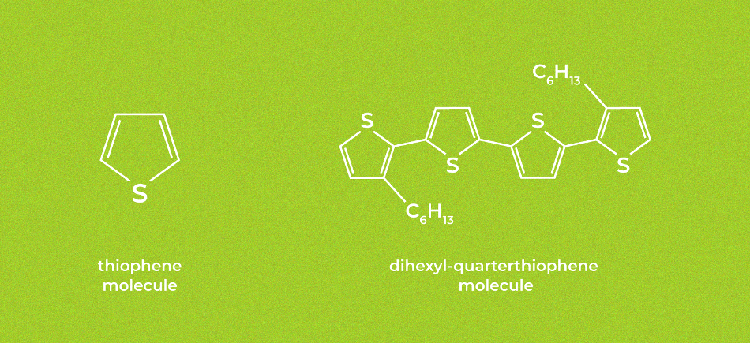 Structural formulas of the thiophene and dihexyl-quarterthiophene molecules. (Image credit: Elena Khavina/MIPT Press Office)
Structural formulas of the thiophene and dihexyl-quarterthiophene molecules. (Image credit: Elena Khavina/MIPT Press Office)
DH4T thin films are organic semiconductors with potential for use in flexible electronics. It was discovered that as soon as the thin films go through a transition from the crystal state to the liquid-crystal state, they tend to lose part of their electrical conductivity.
In addition, the researchers found a “third phase” that does not take place in bulk material and matches with the semiconductor’s monomolecular layer. This kind of structure may be conducive for charge transport across the thin films, with promising implications for microelectronics design. The results of the study have been reported in Nanoscale Research Letters.
Oligothiophenes have been shown to be potential organic semiconductors. The rod-shaped molecules of these materials can orient at the surface on which they have been introduced, piling up cycles of hydrocarbons comprising a sulfur atom called thiophenes, similar to stacks of coins. A herringbone pattern is formed by the “coin edges” in the adjacent stacks. As a result of this molecular arrangement, the charge transfer takes place from one molecule to the other.
The increased number of thiophenes in the molecule translates to increased electrical conductivity, at the cost of the solubility of the compound. The optimal number of the so-called thiophene moieties is believed to be four. Solubility is increased by grafting hexyl fragments to the ends of the conjugated molecular fragment.
DH4T was initially dissolved and evaporated in a vacuum reactor and the material was subsequently deposited as thin films on a silicon substrate. Then, with the help of grazing-incidence X-ray diffraction, the samples’ crystal structure was examined.
In this method, a film is exposed to X-rays at an extremely small glancing angle to increase the distance traveled by the X-ray beam in the film, undergoing many reflections. If not, the signal emitted from the thin film would be extremely feeble and may not be distinguished from the signal emitted from the substrate.
Through the diffraction measurements, the researchers were able to detect the arrangement of molecules in the material piled on the substrate.
At first, DH4T was extremely crystalline. The molecules of this material created a herringbone pattern and they were positioned nearly perpendicular to the substrate. Conversely, as soon as the material was heated to 85 ºC, it went through a phase transition—there was a change in the molecular arrangement, which resulted in the formation of a liquid crystal phase and reduced the films’ electrical conductivity.
The sample was additionally heated to 130 ºC and then cooled to room temperature. This partially restored the crystallinity of the material and thus conductivity.
A third structure appeared in the X-ray diffraction profile during the course of heating. This was specified by weak diffraction maxima that did not correspond with the liquid crystal phase. Previous studies have associated such maxima with monolayers of compounds, for example, DH4T. An interesting fact was this “third phase” was also detected at 70 ºC.
The structure of the monolayer identified by the researchers is conducive for charge transport along the film’s plane, rendering it important for use in flexible electronics. In addition to that, the recently observed phase can also take place in the thin films of other kinds of compounds, the structure of which is analogous to that of DH4T.
Materials like that are utilized in microelectronics. Since the charge is mainly transferred in an extremely thin layer close to the substrate, the team’s findings indicate the need to account for the way the nanostructure of the material has an impact on its conductivity.
Professor Dimitri Ivanov is the director of research at the French National Center for Scientific Research (CNRS) and heads the Laboratory of Functional Organic and Hybrid Materials at MIPT. He co-authored the latest study and commented on its findings: “Using in situ methods, such as structural analysis, and at the same time measuring sample electrical properties enables us to gain insights into the nature of complex phase transitions in the material and assess its potential for practical applications in organic electronics.”