Jul 31 2019
In the latest developments in solar cell technology, polycrystalline perovskite films are used as the active layer, with the efficiency increased as high as 24.2%.
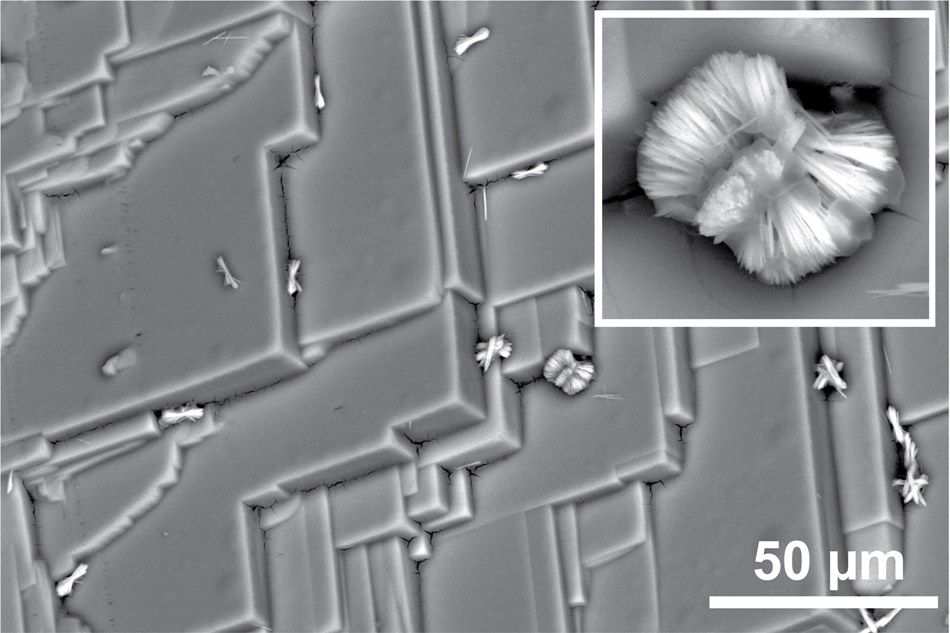 Electron microscopy image of the strongly restructured crystal surface after treatment by benzylamine. On top of the etched 3D crystal, traces of what appears to be the 2D perovskite can be seen. (Image credit: Loi Lab/University of Groningen)
Electron microscopy image of the strongly restructured crystal surface after treatment by benzylamine. On top of the etched 3D crystal, traces of what appears to be the 2D perovskite can be seen. (Image credit: Loi Lab/University of Groningen)
Hybrid organic-inorganic perovskites are shown to be specifically effective, and they have been utilized in optoelectronic devices such as lasers, solar cells, light-emitting diodes, and photodetectors.
However, the surface of the hybrid organic-inorganic perovskites is vulnerable to surface traps, or surface defects, where charge carriers are confined in the semiconducting material. Hence, to overcome this issue and lower the number of traps, the surface of the crystal should be passivated.
Before using the perovskites, the materials can be treated with atmospheric gases, vapors, and chemical solutions to eliminate defects that make them less effective. One specifically effective molecule for this purpose is benzylamine. An in-depth understanding of the chemical and physical mechanisms through which these treatments work is critical for boosting the collection of charge carriers within the solar cells.
The authors have described their work in the new article recently published in AIP Publishing’s Applied Physics Review. They initially tested the hybrid organic-inorganic perovskite crystals that were treated with benzylamine to examine the mechanisms through which the crystal surface is passivated, and the number of trap states is reduced.
This molecule has been used in polycrystalline fields in solar cells, and people have demonstrated that the solar cells were improved, We wanted to study, in a clean system, why the solar cells were improving and understand why adding this molecule makes the devices better.
Maria A. Loi, Professor, University of Groningen
The experiments showed how benzylamine penetrates the crystal surface to produce 2D perovskite, a new, two-dimensional material—on the surface of the three-dimensional (3D) crystal. A terraced etching pattern occurs where the 2D version forms and subsequently breaks away from the crystal surface.
“The main purpose was to passivate the surface to reduce defect states,” Loi stated. “To our surprise, we found out the surface was modified, which was not an expected mechanism. People report that this molecule can improve the quality of devices, but nobody has reported that, in reality, it was creating a two-dimensional layer and could also restructure the material.”
In addition, the authors identified that the combination of atmospheric gases and benzylamine is most effective for passivation. According to Loi, this could mean that more than a single type of trap state exists. Through additional analysis of numerous types of trap states, the mechanisms involved in crystal preparation can be accurately adjusted for efficient optoelectronic devices.