Oct 10 2019
Scientists have discovered a new method to make a polymer material known as polybenzoxazole (PBO), which is commercially known as Zylon. This material is used in the manufacture of bulletproof vests and other high-performance fabrics.
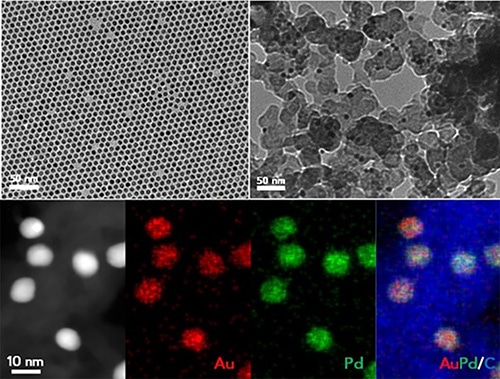 The new catalysts are made from alloyed nanoparticles of gold (Au) and palladium (Pd). (Image credit: Brown University)
The new catalysts are made from alloyed nanoparticles of gold (Au) and palladium (Pd). (Image credit: Brown University)
The new method could be beneficial in creating PBO products that are resistant to degradation, an issue that has affected PBO-based materials even earlier.
We show that using a nanoparticle catalyst, we can produce PBO in more environmentally friendly conditions and without using a chemical that’s known to cause these materials to degrade unexpectedly. We think this could be a path toward making more robust PBO materials.
Shouheng Sun, Study Co-Author and Professor of Chemistry, Brown University
The study has been reported in the journal Matter.
The conventional method to produce PBO requires the use of polyphosphoric acid (PPA) both as a solvent and a catalyst for basic chemical reactions. PPA—a powerful and extremely corrosive acid—has been identified as the source of PBO degradation.
Molecules of the acid tend to get trapped in the polymer chain, making the fibers vulnerable to degradation upon exposure to moisture and light over time. In the past, this degradation caused the recall of PBO-based body armor.
Sun’s lab at Brown University has been largely working with composite nanoparticle catalysts that can activate the new reactions needed to produce PBO, and they achieve that without adding PPA. Catalysis of the reactions using nanoparticles would also necessitate less energy and can be carried out using renewable formic acid as a hydrogen source. All of that renders the manufacturing process more environmentally friendly.
However, so far, composite nanoparticle catalysts have mostly been used to develop only tiny organic molecules. It was not known earlier whether a composite catalyst, which in this case is composed of particles of palladium and gold alloys, could be applied to catalyze the controlled growth of polymer chains.
The key question we were trying to answer is if we can control the reactions so that we get a good control on the degree of polymerization. We ultimately showed that we could do that by tuning the composition and size of the alloy nanoparticles in our catalyst.
Shouheng Sun, Study Co-Author and Professor of Chemistry, Brown University
An alloy composition of almost 60% palladium and 40% gold was demonstrated to be ideal for regulating the rate of reactions required to produce PBO. Particles with a size of about 8 nm led to a reaction speed that maximized the PBO polymers’ molecular weight.
The researchers identified whether the PBO was actually resistant to degradation by performing mechanical testing in collaboration with scientists from Brown’s School of Engineering. The tests revealed that the PBO polymers composed of the nanoparticle catalyst were more resistant to degradation compared to commercial Zylon—even after being boiled in acid and water for several days.
The scientists state that ongoing study will emphasize on producing PBO polymers with greater molecular weights. The polymers produced for this research were considerably lighter than commercial-grade products, which restricts their original mechanical strength. Yet, according to the research team, the study is a solid proof-of-concept for the concept that composite nanoparticles can be used to develop degradation-resistant PBO.
Jerome Robinson, an assistant professor of chemistry at Brown and co-author of the paper, observed that the diversified expertise of the Brown study team was crucial to the success of this research.
“It was really important that we were able to collaborate with engineers and other researchers,” stated Robinson. “To be able to walk across the street to the School of Engineering and do the mechanical testing was great, and I think we have the right team to carry this research forward.”
Chao Yu, Xuefeng Guo, Zhouyang Yin, Zhonglong Zhao, Xing Li, Michelle Muzzio, Cintia Barbosa, Mengqi Shen, Yucheng Yuan, Junyu Wang, John Antolik, Gang Lu, Dong Su, Ou Chen, Pradeep Guduru, and Christopher Seto were the other co-authors of the study.
The study’s early stage was aided by the U.S. Army Research Laboratory and the U.S. Army Research Office (W911NF-15-1-0147). Further support was given by Brown’s Office of the Vice President for Research and the Institute of Molecular and Nanoscale Innovation.