Picosun Group, the leading supplier of AGILE ALD® (Atomic Layer Deposition) thin film coating solutions for global industries, expands its selection of biocompatible ALD materials to be used in medical applications.
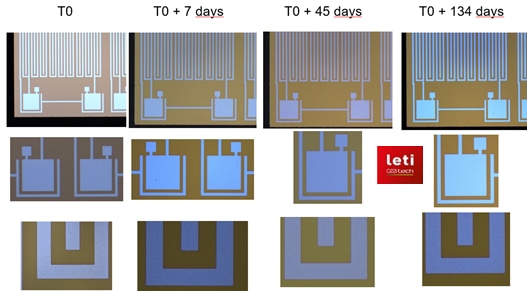 Microimplant electronics protected by Picosun’s ALD HfO2. No changes after soaking in 87 oC PBS for over 3 months which correlates to over 10 years in human body. T0 = starting point of the test. Reference: InForMed project, image source CEA-Leti.
Microimplant electronics protected by Picosun’s ALD HfO2. No changes after soaking in 87 oC PBS for over 3 months which correlates to over 10 years in human body. T0 = starting point of the test. Reference: InForMed project, image source CEA-Leti.
Picosun’s TiO2 and Al2O3 processes are already used in production of surgical implants and in drug particle coating for controlled drug delivery. Now, also HfO2, SiO2, ZrO2, Nb2O5, Ta2O5, AlN and TiN ALD films manufactured by Picosun have been tested and validated by an independent third party to be non-cytotoxic and safe to human tissues in e.g. implant applications (*).
This wide variety of materials gives great flexibility in designing novel ALD solutions for a plethora of healthcare uses, when the materials can be used either as such, or combined into nanolaminates or doped films with unique, application-wise tailorable physico-chemical properties (**).
ALD, with its innate ability to create ultra-thin material layers with the highest conformality, uniformity, and structural quality, has enormous potential to solve various key issues in medical applications where implantable devices are involved. Orthopaedic implants, pacemakers, implantable hearing or eyesight aids, microimplants for sensing, monitoring and analysis applications, and brain or heart probes for therapeutic or diagnostic uses all contain parts that are sensitive to the corrosive environment of the human body. Protective encapsulation of these devices is thus needed to ensure their correct operation, long enough operational lifetime, and also to protect the body from the possible rejection reaction or contaminant leakage from the devices’ corroding parts. Various polymer layers have typically been used as encapsulants, but their downside is their thickness and robustness which unnecessarily increases the mass and dimensions of the implant.
Compared to polymer encapsulation, ALD offers a truly elegant, sophisticated solution to implant manufacturers. Practically massless and invisible, but still dense, flexible, pinhole- and crack-free ALD thin films cover reliably even the smallest microscale surface features of the coated object, they can be applied at moderate temperatures, and – as now analysed in medical industry’s standard tests – several ALD materials are intrinsically biocompatible. As ALD is a mature, key enabling technology in semiconductor and microelectronics manufacturing for decades already, the processes and practises for industrial introduction and ramp-up exist, and can be readily applied to new fields as well.
”Healthcare sector is one of our key strategic directions. Our patented know-how of ALD-based biocompatible protective encapsulation for implantable medical devices has already raised significant interest amongst industry leading companies. We are pleased that we have now even wider portfolio of materials and solutions that we can provide to these companies. Not only can our ALD technology solve several challenges these industries are currently facing, but also enable completely new components and devices to realize future’s healthcare inventions,” states Dr. Jani Kivioja, CTO of Picosun Group.
(*) FICAM – The Faculty of Medicine and Health Technology, University of Tampere, Finland: Cytotoxicity tests with cell culture medium according to the ISO 10993-5 standard, and 3 weeks soaking tests in PBS (phosphate-buffered saline) at 87 oC.
(**) Examples of Picosun’s ALD materials’ performance in implant encapsulation.