Jan 27 2020
Researchers at the University of California, Berkeley (UC Berkeley) have developed a blue light-emitting diode (LED) from a new, popular semiconductor material called halide perovskite. This latest development overcomes a major obstacle to using these easy-to-make and low-cost materials in electronic devices.
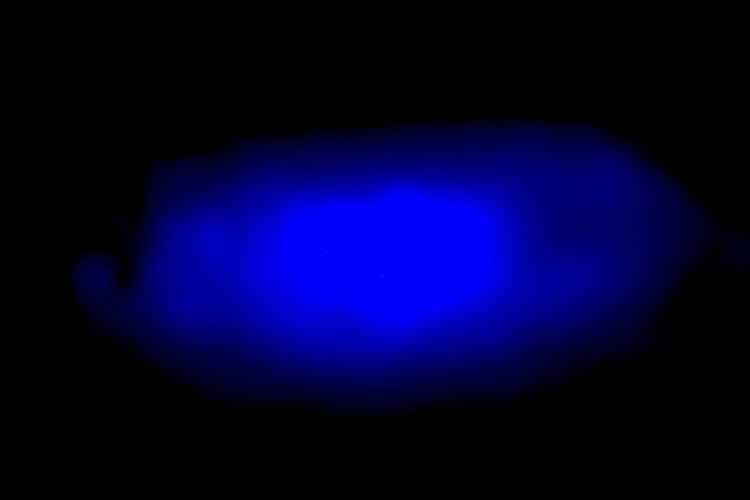 UC Berkeley chemists created a type of halide perovskite crystal that emits blue light, something that has been hard to achieve with the trendy new material. But the researchers also found that these materials are inherently unstable, requiring careful control of temperature and chemical environment to maintain their precise color. Image Credit: Peidong Yang.
UC Berkeley chemists created a type of halide perovskite crystal that emits blue light, something that has been hard to achieve with the trendy new material. But the researchers also found that these materials are inherently unstable, requiring careful control of temperature and chemical environment to maintain their precise color. Image Credit: Peidong Yang.
But in the process, the scientists identified an underlying characteristic of halide perovskites that may prevent them from being extensively used as transistors and solar cells. Moreover, this special property may pave the way for a whole new world for perovskites that significantly surpasses the property of existing standard semiconductors.
In a paper that appeared in the Science Advances journal on January 24th, 2020, Peidong Yang, a chemist at UC Berkeley, and his collaborators demonstrated that the chemical environment, humidity, and temperature cause changes in the crystal structure of the halide perovskites, disrupting their electronic and optical characteristics.
Perovskite devices are innately unstable because the chemical and physical environments are not closely regulated. This does not pose a major issue for conventional semiconductors.
Some people may say this is a limitation. For me, this is a great opportunity. This is new physics: a new class of semiconductors that can be readily reconfigured, depending on what sort of environment you put them in. They could be a really good sensor, maybe a really good photoconductor, because they will be very sensitive in their response to light and chemicals.
Peidong Yang, S. K. and Angela Chan Distinguished Chair in Energy, College of Chemistry, University of California, Berkeley
Yang is also the director of the Kavli Energy NanoSciences Institute.
Today’s silicon- or gallium nitride-based semiconductors are extremely stable across a broad range of temperatures. This is mainly because powerful covalent bonds hold the crystal structures of the semiconductors together. Weaker ionic bonds hold halide perovskite crystals together, just like those in a salt crystal.
This implies that halide perovskite crystals can be made more easily; however, while they can be evaporated out of a basic solution, they are sensitive to heat, humidity, and other environmental conditions.
This paper is not just about showing off that we made this blue LED. We are also telling people that we really need to pay attention to the structural evolution of perovskites during the device operation, any time you drive these perovskites with an electrical current, whether it is an LED, a solar cell or a transistor.
Peidong Yang, S. K. and Angela Chan Distinguished Chair in Energy, College of Chemistry, University of California, Berkeley
Yang continued, “This is an intrinsic property of this new class of semiconductor and affects any potential optoelectronic device in the future using this class of material.”
Yang is also a professor of materials science and engineering at UC Berkeley and a senior faculty scientist at Lawrence Berkeley National Laboratory (Berkeley Lab).
The Blue Diode Blues
According to Yang, it has always been a challenge to make semiconductor diodes that produce blue light. The breakthrough development of efficient blue LEDs from gallium nitride received the Nobel Prize for Physics in 2014.
Diodes are the optoelectronic components incorporated in fiber-optic circuits and in general-purpose LED lights. They produce light upon applying electric current.
In 2009, halide perovskites attracted a great deal of attention, when researchers in Japan discovered that these materials make highly efficient solar cells. Since that time, these low-cost and easy-to-make crystals have captured the interest of scientists. To date, only green and red LEDs have been shown and not blue.
Halide perovskite blue-LEDs have not been stable—that is, their color changes to redder and longer wavelengths with use.
As Yang and his collaborators found, this phenomenon is attributed to the special nature of the crystal structure of perovskites. Halide perovskites are made up of a metal, like tin or lead, thrice the number of halide atoms, like iodine, bromine, or chlorine, and equal numbers of larger atoms, like cesium.
When all these elements are combined together in a solution and subsequently dried, the atoms arrange into a crystal, just like how salt crystallizes from seawater.
With the help of a novel method together with the ingredients like bromine, lead, and cesium, chemists at the UC Berkeley and Berkeley Lab developed perovskite crystals that produce blue light. They subsequently bombarded these crystals with X-rays at the Stanford Linear Accelerator Center (SLAC) to establish their crystalline structure at different temperatures.
The chemists discovered that when the crystal was heated from room temperature (approximately 300 K) to about 450 K—which is a standard operating temperature for semiconductors—the squashed structure of the crystal expanded and ultimately formed into a novel tetragonal or orthorhombic configuration.
Since the arrangement of atoms and the distances between them control the light produced by these crystals, the color also changes with temperature. A perovskite crystal that produced blue light (450 nm wavelength) at 300 K abruptly produces blue-green light at 450 K.
According to Yang, the flexible crystal structure of the perovskites is caused by the weaker ionic bonds that are characteristic of halide atoms. Mineral perovskite that occurs naturally incorporates oxygen rather than halides and creates a highly stable mineral.
Gallium nitride semiconductors and silicon-based semiconductors are analogously stable because the atoms are joined by powerful covalent bonds.
Making Blue-Emitting Perovskites
Yang stated that it is difficult to produce blue-emitting perovskite diodes because the typical method used for growing the crystals as a thin film promotes the formation of a combination of crystal structures, with each emitting at a different wavelength.
Electrons move rapidly toward those crystals that have the tiniest bandgap—that is, the tiniest range of unallowed energies—and then produce light, which happens to be red.
To prevent this, Yang’s postdoctoral fellows as well as the study’s co-first authors—Hong Chen, Jia Lin, and Joohoon Kang—developed one-layered crystals of perovskite. Then, using a low-tech technique to produce graphene, they utilized tape to remove just one uniform perovskite layer. The perovskite shined blue when it was integrated into a circuit and fed with electricity.
The real blue wavelength differed with the number of octahedral perovskite crystal layers. These crystals are separated from each other by an organic molecular layer that enables the perovskite layers to be easily separated and also safeguards the surface.
However, the SLAC experiments demonstrated that perovskites that emit blue light altered their emission colors with temperature. This characteristic can have fascinating applications, added Yang.
A couple of years ago, Yang demonstrated a window composed of halide perovskite that becomes transparent when the sun goes down and becomes dark in the presence of the sun. It also generates photovoltaic energy.
We need to think in different ways of using this class of semiconductor. We should not put halide perovskites into the same application environment as a traditional covalent semiconductor, like silicon. We need to realize that this class of material has intrinsic structural properties that make it ready to reconfigure. We should utilize that.
Peidong Yang, S. K. and Angela Chan Distinguished Chair in Energy, College of Chemistry, University of California, Berkeley
The study was funded by the U.S. Department of Energy’s Basic Energy Sciences program. The study’s co-authors are Qiao Kong, Dylan Lu, Minliang Lai, Li Na Quan, and Jianbo Jin from UC Berkeley; Jun Kang, Zhenni Lin, and Lin-wang Wang from Berkeley Lab; and Michael Toney from SLAC.
Chen is presently at Southern University of Science and Technology based in Shenzhen, China; Lin is working at Shanghai University of Electric Power; and Joohoon Kang is based at Sungkyunkwan University located in Seoul, South Korea.