Methane—a component of natural gas—is found in large amounts in the earth’s crust. It has been found to be potential in present-day applications, chiefly as burning fuel.
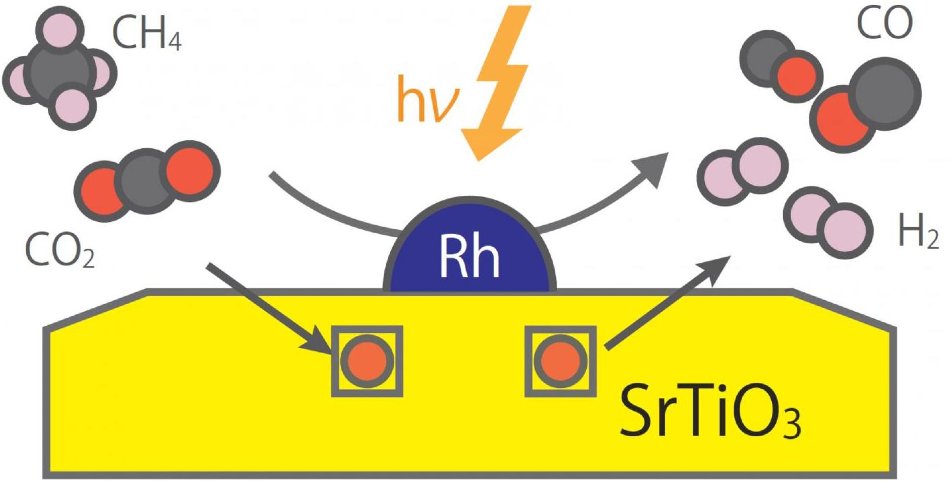 Strontium titanate combined with rhodium nanoparticles converted methane and carbon dioxide into synthesis gas under light irradiation at much lower temperatures than those required in thermal reactors. Image Credit: Tokyo Tech.
Strontium titanate combined with rhodium nanoparticles converted methane and carbon dioxide into synthesis gas under light irradiation at much lower temperatures than those required in thermal reactors. Image Credit: Tokyo Tech.
Instead, it can be transformed into a useful blend of carbon monoxide and hydrogen, known as “synthesis gas,” formed by reaction with carbon dioxide. This process is known as dry reforming of methane (DRM).
This DRM reaction involves the use of external energy; therefore, it is called “uphill.” In this case, the thermal reactors should be maintained at a temperature of 800 °C and above for effective conversion.
This can only be achieved by burning other fuels, which leads to enormous greenhouse gas emissions, the main cause of climate change. Moreover, high temperatures used can lead to the deactivation of widely used catalysts, due to carbon precipitation (or coking) and aggregation.
Rather than addressing such limitations of thermal catalysis systems for DRM reaction, scientists have now made efforts to use photocatalysts activated by light to manage methane conversion at considerably lower temperatures. Despite the fact that many photocatalyst-like materials have been put forward, achieving an acceptable conversion performance at low temperatures has been difficult.
Luckily, scientists, including Prof. Mashiro Miyauchi, found a potential combination of materials that can serve as a potent photocatalyst in the reaction for converting methane into synthesis gas.
Most notably, they found that a combination of strontium titanate and rhodium nanoparticles facilitated the conversion of carbon dioxide and methane into synthesis gas, by irradiation with light, at considerably lower temperatures than those needed in the thermal reactors.
The scientists found that the proposed photocatalyst was more stable than already tested catalysts and even prevented other problems like coking (“sooting”) and aggregation (clumping) of the catalyst particles. Above all, as explained by Prof. Miyauchi, “The proposed photocatalyst allowed us to vastly surpass the limitations of thermal catalysts, yielding high performance for synthetic gas production.”
The scientists also explained how the proposed photocatalyst induces an improved methane conversion through physical mechanisms. This understanding is largely crucial because of the uses it has for different types of methane reactions. The present system needs irradiation by ultraviolet (UV) light, which is just a small percentage of solar light.
The present study provides a strategic way to perform uphill reactions using methane and creates a connection between the fossil fuel industry and renewable energy applications. Now we are developing the visible-light-sensitive system.
Mashiro Miyauchi, Professor, Tokyo Institute of Technology
Hopefully, the study outcomes will pave the way for more environmentally friendly developments and help lower carbon emissions in the future.