Jan 29 2020
Whenever a new kind of material had to be developed, engineers or chemists would simply go to the laboratory and begin “cooking.” Just like attempting to enhance upon food recipe, the process of making a material involves experimentation with novel chemical ingredients or adjustments to the durations and temperatures of cooking.
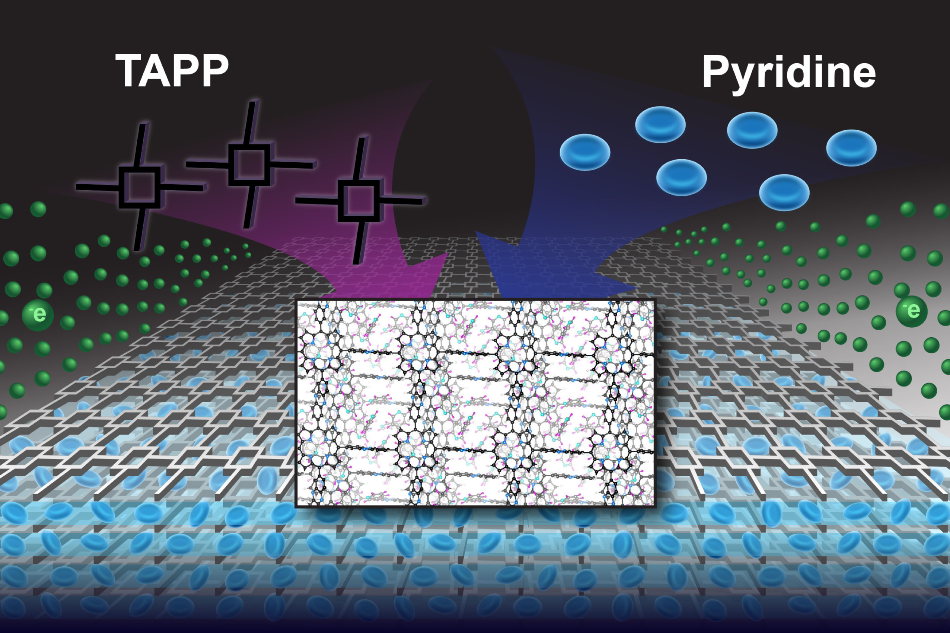 A graphical representation of the covalent organic frameworks, or COFs, created by a collaborative team of experimental and theoretical chemists. Large porphyrin structures (labeled as TAPP) form an egg carton-like lattice that forms multiple stacks, with pyridine molecules (shown in blue) filling the spaces in between the layers. An electrical current is depicted in green. Image Credit: Felice Macera.
A graphical representation of the covalent organic frameworks, or COFs, created by a collaborative team of experimental and theoretical chemists. Large porphyrin structures (labeled as TAPP) form an egg carton-like lattice that forms multiple stacks, with pyridine molecules (shown in blue) filling the spaces in between the layers. An electrical current is depicted in green. Image Credit: Felice Macera.
But instead of depending on a lengthy process that offers no guarantee of success, what if researchers create a new material by simply “snapping” different chemical “pieces” together?
A research team from the University of Nebraska–Lincoln (UNL), the University of Pennsylvania, Harbin Institute of Technology in China, and Colorado School of Mines, has demonstrated a new method for producing organic “Legos” that can be simply joined together to create novel materials.
A framework like this produces porous and lightweight structures that can be rapidly synthesized and easily altered to produce novel materials that have special properties. The study was published in the Journal of the American Chemical Society.
The latest study focuses on a comparatively novel structure called covalent organic frameworks, or COFs, for short. COFs are two-dimensional (2D) and three-dimensional (3D) organic solids that are held together with powerful covalent bonds. They have crystalline structures composed of light elements like oxygen, nitrogen, and carbon, which make them durable and lightweight.
Individual chemical building blocks, just like individual Lego pieces, can be arranged in defined ways to create a bigger structure that can be planned in excellent detail rather than adding components to a mixture and waiting to see what comes out.
The particular building blocks utilized in this analysis are called porphyrins, which are a group of organic structures present in proteins like chlorophyll and hemoglobin. These organic structures contain a reactive metal atom at their core, which scientists would like to use to develop COF materials that have improved properties.
However, in spite of the broad range of promising applications, which span from carbon capture to hydrogen storage, these COF materials have practical restrictions. It takes a long time to create COFs, and it can take many days to produce just 1 g of material. Moreover, current techniques can only create the powder form of COFs, making it relatively harder to process or transfer these materials onto other kinds of materials.
With the UNL researchers leveraging their expertise in electropolymerization—a technique used to regulate the synthesis of polymers on an electrically conductive substrate—the scientists discovered that electricity can potentially be used to produce thin films of COFs.
The ensuing material, that is, 2D sheets arranged in numerous layers, is heat-tolerant, lightweight, and takes only hours to develop rather than days.
This method is fast, simple and cheap, and you enable deposition of a thin film onto a variety of conductive substrates. Through this approach, we can avoid the common challenges with the COF synthesis through conventional solvothermal method.
Elham Tavakoli, Study Lead, University of Nebraska–Lincoln
Tavakoli performed the study in association with fellow graduate student Shayan Kaviani at UNL, under the guidance of Siamak Nejati, an assistant professor.
However, after thoroughly analyzing the structure of the deposited COFs, the scientists came across something that they could not explain—the distance of the 2D sheets from each other, or the interlayer distances, was relatively larger than anticipated. Following this, the scientists sought the help of theoretical chemists at the University of Pennsylvania to find out what was going on.
After attempting to develop a theoretical model that would precisely elucidate the structure of the COF, Arvin Kakekhani, a postdoc at the University of Pennsylvania, realized that the new model might be lacking something. Kakekhani subsequently examined the list of all the chemicals utilized in the COF synthesis process to check if any of the additives can potentially shed some light on the surprising results.
The scientists were amazed to find that a “spectator” molecule—which they believed only offered the electrochemical setting required to trigger the reaction—was a crucial component of the structure of the COF.
The theory that a pyridine molecule, a tiny organic molecule that has a simple ring structure, can help in the formation of crystals is not a new concept in the field of chemistry, but it was not assumed to be crucial for the COF structure prior to this analysis. The team has now gained a deeper understanding of the way this spectator molecule fits optimally inside the 2D layers and offers the required support for the COFs to assume a crystal structure.
“These smaller pyridine molecules actually go into the material and become part of the crystal,” added Kakekhani.
Now, this novel method is a starting point for developing different kinds of materials. By substituting the pyridine molecule with another tiny one and by altering the kind of COF building blocks used and the reaction conditions, the scope for producing novel materials with special characteristics are unlimited.
COFs are not that old, so they have lots of undiscovered points. I’m looking forward to finding more of these myths in this field.
Elham Tavakoli, Study Lead, University of Nebraska–Lincoln
In the coming days, the team is hoping to improve the catalytic characteristics of the developed COFs and create site-isolated catalysts. These catalysts are substances that boost the speed of a chemical reaction and are important components of industrial processes.
Our current COF has chemical reactivity, but that can be greatly heightened through small modifications. Our team can take one platform and make many materials with different functionalities, all based on the work reported here.
Andrew M. Rappe, Blanchard Professor of Chemistry, School of Arts and Sciences, University of Pennsylvania
“We foresee that the developed platform will allow us to design and realize many functional interfaces not yet explored. A wide range of applications, such as high selectivity separation and efficient catalysis, can be envisioned for these systems,” stated Nejati.
Kakekhani further underscored that the study also demonstrates the significance of having experimentalists and theorists work in close association.
“It was not only about to have something that matches their data,” he added, “but about generating some insight that can make these materials better. It takes two to tango, and if we find a way to use each other’s insight, there is room for discovering new things.”
The study’s authors are Arvin Kakekhani and Andrew M. Rappe from the University of Pennsylvania; Elham Tavakoli, Shayan Kaviani, Mahdi Mohammadi Chaleni, and Siamak Nejati from the University of Nebraska–Lincoln; Mohsen Asle Zaeem from the Colorado School of Mines; and Peng Tan from the Harbin Institute of Technology in China.
The study was funded by the U.S. Department of Energy Office of Basic Energy Sciences Grant DE- SC0019281 and National Science Foundation Grant ECCS-1542182.