Mar 18 2020
The surface of semiconductor materials contains crystal structures capable of making the materials act like metals and also like superconductors. This was revealed by a collaborative research group from Swansea University and the University of Rostock.
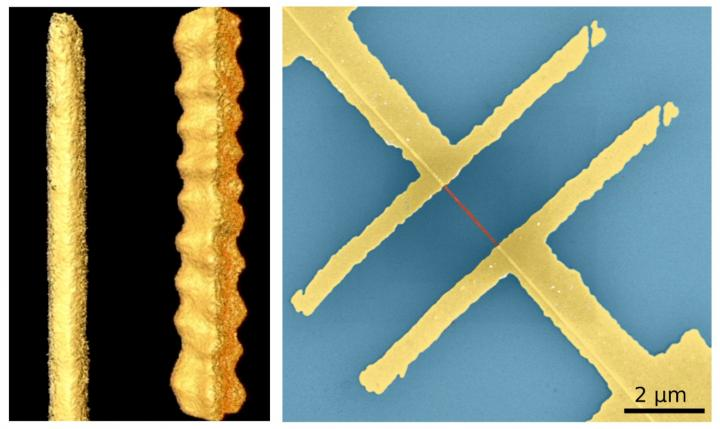 Left: Shape of nanostructures made of lead sulfide, computer reconstructed based on a series of transmission electron microscopy images. The left straight stripe behaves like a semiconductor and the right zigzag nanowire behaves like a metal. Right: Electrical device consisting of two gold electrodes contacting a nanowire (in red) on a silicon chip (in blue). Image Credit: Hungria/Universidad de Cádiz, Ramin/DESY, Klinke/University of Rostock and Swansea University.
Left: Shape of nanostructures made of lead sulfide, computer reconstructed based on a series of transmission electron microscopy images. The left straight stripe behaves like a semiconductor and the right zigzag nanowire behaves like a metal. Right: Electrical device consisting of two gold electrodes contacting a nanowire (in red) on a silicon chip (in blue). Image Credit: Hungria/Universidad de Cádiz, Ramin/DESY, Klinke/University of Rostock and Swansea University.
The finding could pave the way to advances such as highly energy-efficient electronic devices.
The active parts of different components like integrated circuits, transistors, LEDs, and sensors are semiconductors. Such materials are mainly based on silicon, and they form the core of today’s electronics sector.
Products made of semiconductors are constantly used in computers (as illumination elements), in modern TV sets, and most certainly in mobile phones. However, metals are used for wiring the active electronic components and form the framework of the devices.
Under the guidance of Professor Christian Klinke of Swansea University’s chemistry department and the University of Rostock in Germany, a group of researchers investigated the crystals at the surface of semiconductor materials.
The researchers applied a technique known as colloidal synthesis to lead sulfide nanowires and demonstrated that it is possible to arrange the sulfur and lead atoms constituting the crystals in several ways. Most importantly, they also observed that this process affected the properties of the material.
In a majority of the configurations, the two types of atoms combine and the entire structure exhibits semiconducting behavior, as predicted.
But the group identified that one specific “cut” into the crystal, with the so-called {111} facets on the surface (including only lead atoms), exhibits metallic property.
This implies that the nanowires transport considerably higher currents, their transistor-like behavior is inhibited, they do not react to illumination (as semiconductors do), and exhibit inverse temperature dependency, characteristic of metals.
After we discovered that we could synthesize lead sulphide nanowires with different facets, which makes them look like straight or zigzag wires, we thought that this must have interesting consequences for their electronic properties. But these two behaviours were quite a surprise to us. Thus, we started to investigate the consequences of the shape in more detail.
Dr Mehdi Ramin, Researcher, DESY
Then, the research group made a second discovery, which showed that at low temperatures, the surface of the nanostructures even acts as a superconductor. This implies that the electrons flow through the structures with considerably lower resistance.
This behavior is astonishing and certainly needs to be further studied in much more detail. But it already gives new exciting insights into how the same material can possess different fundamental physical properties depending on its structure and what might be possible in the future.
Christian Klinke, Study Lead Author and Professor, Swansea University and Rostock University
Klinke added, “One potential application is lossless energy transport, which means that no energy is wasted. Through further optimization and transfer of the principle to other materials, significant advances can be made, which might lead to new efficient electronic devices.”
“The results presented in the article are merely a first step in what will surely be a long and fruitful journey towards new thrilling chemistry and physics of materials,” concluded Klinke.
The study was published in the Advanced Functional Materials journal.