Oct 12 2020
RUDN University chemists have proposed a new reaction to synthesize organic compounds in one vessel. The final products are effective against the cells of carcinomas, even cells that are drug-resistant. The new reaction has been explained in the Bioorganic Chemistry journal.
 A team of chemists from RUDN University suggested a new reaction to produce organic compounds in one vessel. The end products turned out to be effective against the cells of carcinomas, including drug-resistant ones. Image Credit: RUDN University.
A team of chemists from RUDN University suggested a new reaction to produce organic compounds in one vessel. The end products turned out to be effective against the cells of carcinomas, including drug-resistant ones. Image Credit: RUDN University.
The synthesis of a number of organic substances is a multistage procedure of step-by-step molecule assembly. Only a single chemical bond forms in each stage. Following each stage, the product is cleaned and used in the following stage. Domino reactions are a sequence of reactions that take place one after another in a single vessel without the need for any extra reagents.
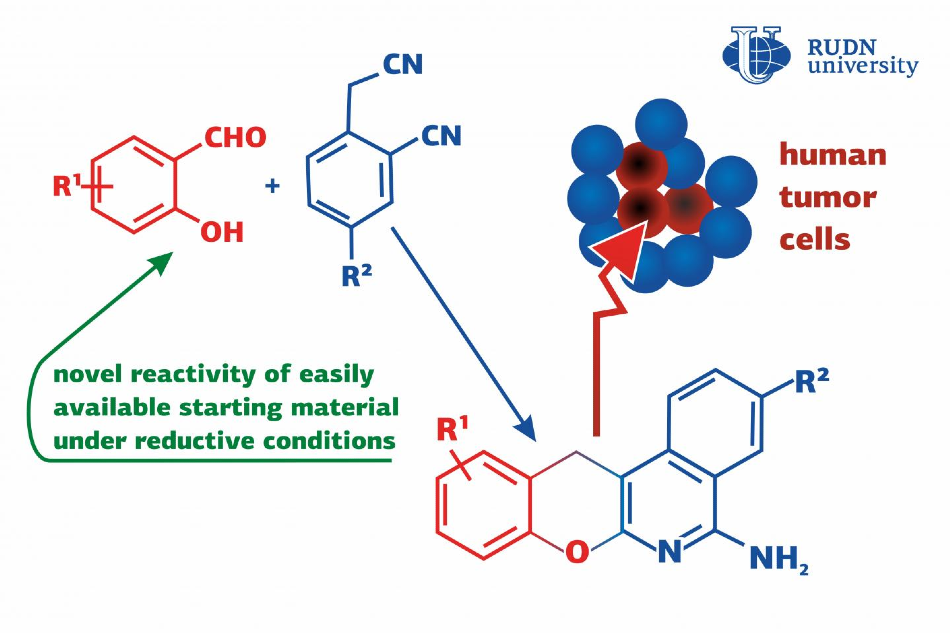
A RUDN chemist team along with the University of Bari determined several substances that trigger a domino synthesis of chromenoisoquinolineamine derivatives. Related compounds are used as antitumor and anti-inflammatory drugs, and a few of them can possibly treat Alzheimer’s disease.
The chemists proposed the use of a derivative of salicylic acid, salicylaldehyde, and homophthalonitrile and catalyzing the reaction with ammonium formate that is economical and environment-friendly. The preliminary reagents were blended with isopropyl alcohol and water and placed inside a microwave reactor where the mixture was heated for 20 minutes at about 150°C.
The team used salicylaldehydes with various substituents and, consequently, produced 19 derivatives of chromenoisoquinolineamines with 43%–85% yields of corresponding reaction products.
To examine the medical prospect of the new substances, the chemists tried out their effect on human cancerous cells. Cisplatin used in chemotherapy and said to destroy tumor cells was used as a standard for comparison. The chemists selected the cells of colon and breast cancers, as well as three strains of ovary cancer cells (two of which were cisplatin-resistant) for the experiment.
The whole range of new substances was found to be lethal for tumor cells, including the resistant strains. The scientists chose two compounds that were established as efficient even in low concentrations and used computer modeling. According to it, the reason for their effectiveness was an extra amine group that develops an unwavering bond with the nucleotides of the cancer cell DNA.
In our work, we searched for new compounds with promising therapeutic properties, as well as for ways to synthesize them. Our approach allows for the synthesis of tumor-combating substances in the course of one domino reaction that is extremely efficient: four new bonds are created within one synthetic operation. We worked together with our Italian partners, and the study was supported by the Russian Science Foundation.
Alexey Festa, Candidate of Chemical Sciences and Senior Lecturer, Department of Organic Chemistry, RUDN University
Festa added, “In the future, we plan to improve our methodology and develop three- and four-component reactions on its basis.”
Journal Reference:
Yue, X., et al. (2020) Reductive domino reaction to access chromeno[2,3-c]isoquinoline-5-amines with antiproliferative activities against human tumor cells. Bioorganic Chemistry. doi.org/10.1016/j.bioorg.2020.104169.