Nov 2 2020
An eco-friendly process for the production of dapsone, a substance that prevents the growth of leprosy and malaria agents, has been proposed by a chemist from RUDN University.
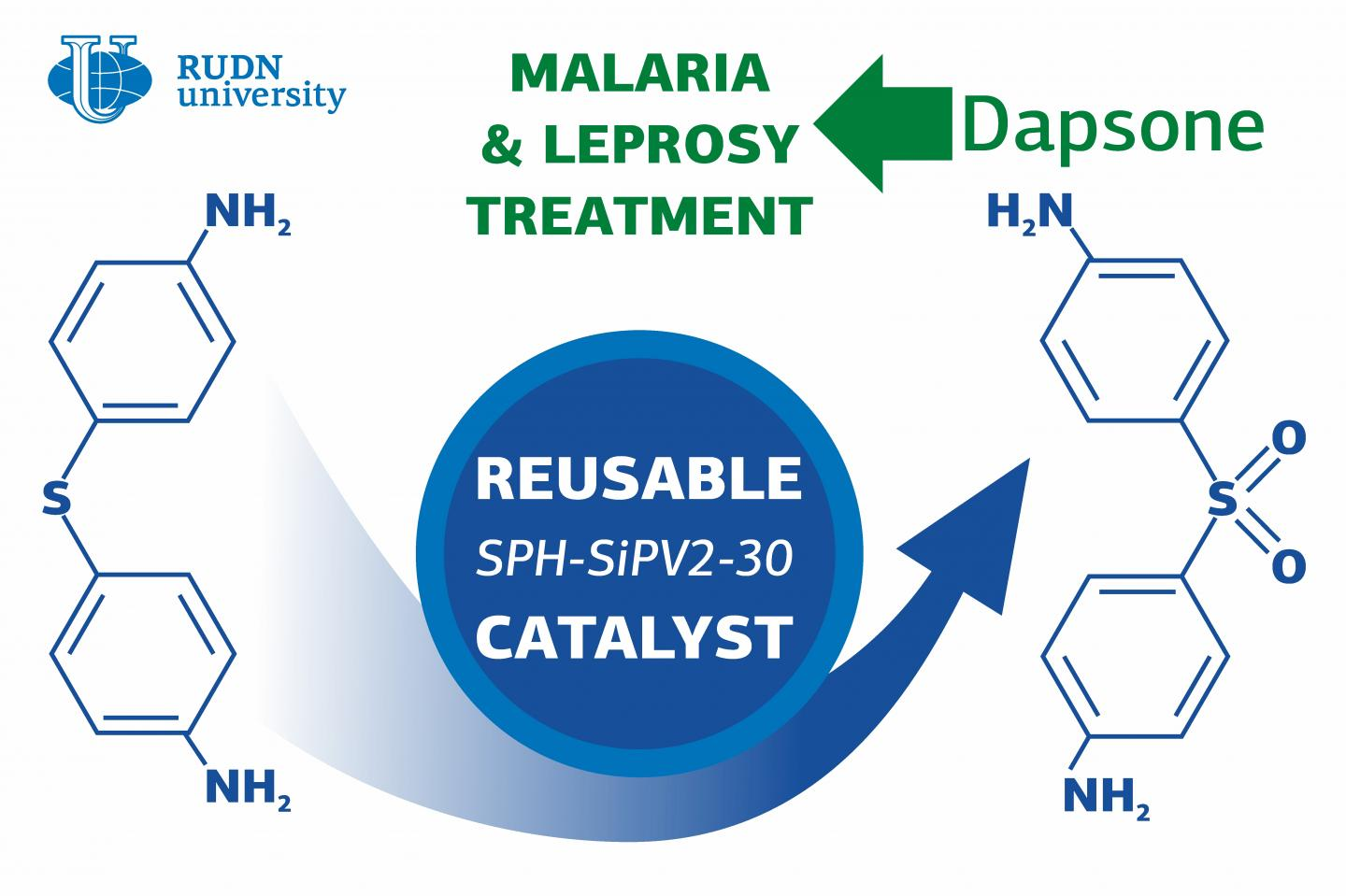
Image Credit: RUDN University.
The key component of the new reaction is hydrogen peroxide that does not create any environmentally harsh compounds, and the only by-product is just water.
In contrast to other technologies, this technique involves only a single stage of dapsone production and does not need high temperatures. The reaction catalyst can be reused without compromising efficiency. The study findings were reported in Microporous and Mesoporous Materials.
It is widely thought that leprosy is a disease of the past, but approximately 200,000 cases are registered in the world (mostly in Brazil, India, and Nepal) each year. It can be treated with antibiotics that stop the growth of the Mycobacterium bacteria. By contrast, malaria is one of the world’s most extensively spread diseases with more than 200 million cases per year.
The spread of its agent (a protist from the genus Plasmodium) also can be blocked by using antibiotics. Dapsone is a safe and easily accessible drug that treats both the diseases and is included in the WHO Model List of Essential Medicines. However, its manufacture is not environment-friendly, as the synthesis reaction necessitates high temperatures and the use of harsh acids (such as sulfuric acid).
A chemist from RUDN University has now proposed a green technology for dapsone production that could possibly help expand its manufacture, such that more patients can access the drug.
Dapsone or diaminophenyl sulfone comprises two benzene rings with NH2 amino groups. The rings are linked with an oxidized atom of sulfur, or an SO2 group. To get dapsone, producers oxidize its precursor wherein the bond between the rings is developed by a sulfur and hydrogen (SH) group.
But oxidation can also impact sensitive amino groups. Thus, they must be protected before the reaction begins, for example, by connecting special protective groups to them.
The RUDN chemist created a catalyst that facilitates the oxidation of SH groups in the precursor with basic hydrogen peroxide. Hydrogen peroxide is said to be the most eco-friendly oxidizing agent because its sole by-product is water. The oxidation reaction occurs at room temperature, includes just one stage, and does not need protection of amino groups.
None of the earlier dapsone synthesis reactions can be called completely environmentally friendly as they happen in rigid conditions and have several stages: adding protective groups, synthesis, and their removal. This complexity increases the chances of by-products that should be removed from the reaction.
Raphael Luke, PhD, Head, Molecular Design and Synthesis of Innovative Compounds for Medicine Science Center, RUDN University
His team created a wolfram-based catalyst from polyoxometalates by substituting a few wolfram atoms with vanadium. This boosted the catalyst’s acidic properties and accelerated the reaction, enabling it to occur even at low temperatures.
The new compound was enclosed in a porous material, a hydrogel composed of acrylamide and propanoic acid, to stop the catalyst from being washed off from the reaction.
This step enables the catalyst to be reused a minimum of three times without any of its efficiency being lost. The researchers also identified the most ideal production conditions and reagent concentration and succeeded in achieving 100% oxidation of the dapsone precursor at 25 °C in just 9 hours.
Journal Reference:
Frenzel, R. A., et al. (2020) A green and reusable catalytic system based on silicopolyoxotungstovanadates incorporated in a polymeric material for the selective oxidation of sulfides to sulfones. Microporous and Mesoporous Materials. doi.org/10.1016/j.micromeso.2020.110584.