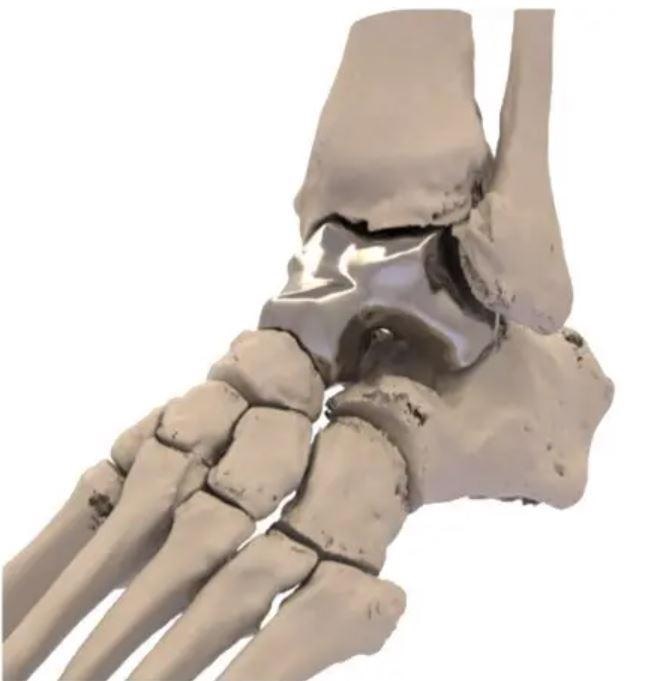
Image Credit: Additive Orthopaedics
As well as offering relief and guaranteeing mobility and pain reduction for thousands, a new 3D-printed metal talus implant could change the field of orthopedics.
For the first time, the FDA has approved a 3D printed metal implant intended to replace bone affected by injury or illness. The talus is the ankle joint’s main bone connecting the leg to the foot. The cobalt-chromium alloy implant is designed by Additive Orthopaedics to combat osteonecrosis. It is otherwise known as avascular necrosis (AVN) — the death of bone tissue due to a lack of blood supply.
The talus replacement developed by the New Jersey-based company is designed by taking a CT scan of a patient’s intact talus bone in their unaffected ankle and using it as a blueprint for 3D printing.
This means a replacement talus that is tailored to the individual patient. As well as this personalization, the 3D-printed metal talus could help patients maintain mobility, granting it a significant benefit over current treatments.
Combating Avascular Necrosis Whilst Preserving Mobility
AVN can be caused by a sudden injury — a broken bone or a dislocated joint — or by damage to tissue over time. The restriction of blood supply causes bone tissue to become necrotic. For joints like the ankle, this means the deterioration of cartilage that prevents bones from rubbing together. In its early stages, this results in pain and arthritis. In its later stages, the talus or even the entire ankle can collapse.
Current treatments for conditions that degrade or damage the talus involve fusing the foot and ankle bones together — a surgical procedure that fixes the joint in place, meaning the ankle can’t be moved.
Until recently this fusion has been the only plausible treatment when the talus bone dies. In most other cases, when this treatment method — itself the cause of a lifetime of limited movement — is not viable, the foot itself has to be amputated.
“Avascular necrosis of the ankle, while a rare condition, is a serious and potentially debilitating one that causes pain and can lead to inhibited motion of the ankle joint, and in some cases, removal of part of the leg,” said Raquel Peat, Ph.D., M.P.H., USPHS, and director of the FDA’s Center for Devices and Radiological Health’s Office of Orthopedic Devices. “Today’s action provides patients with a treatment option that could potentially reduce pain, retain a range of motion of their joint, and improve quality of life.”
The metal replacement talus could, therefore, offer hope to those with this condition without having to undergo surgery.
Why the Talus?
With 206 bones in the human body and 26 in the foot alone — many of which are also affected by degenerative disorders — the talus may seem like an odd choice to start integrating metal replacement devices.
The reason for selecting this ankle bone hinges on the fact that it is crucial for transferring weight from the leg to the foot and the bone suffers from limited blood circulation even before injury or illness. This means a traumatic injury of an illness like sickle cell or leukemia that can further restrict blood flow can quickly cause the talus to die.
There are currently a few teams offering to replace the talus with a 3D-printed metal counterpart. Dr. Dane Wukich is part of the Department of Orthopedic Surgery at UT Southwestern, one of the few crews in Texas that are providing such medical intervention at the moment.
“The results are truly impressive. Replacing the talus with a 3D replica allows for movement of the ankle and subtalar joints, which makes moving the foot up and down and side to side possible,” says Wukich. “I encourage my patients to return to an activity level with which they are comfortable. While a marathon may not be in the cards, I’ve seen my patients go back to active lives of working, chasing children, and participating in exercise classes.”
The Tip of the Iceberg for 3D Printed Metal Bones
The FDA has granted Additive Orthopaedic’s device a humanitarian use exemption classifying it as a Humanitarian Use Device (HUD). This means that the team did not technically have to provide clinical evidence of the device’s efficacy.
Fortunately, such evidence would have been available should it have been required thanks to a clinical trial that provided 31 subjects with 32 replacement talus devices, as one patient required a replacement in both ankles.
The study found pain reduced for the subjects over a period of 3 years following the replacement, with only three patients needing further surgery.
The humanitarian use exemption was granted to the team due to the fact that less than 8,000 people per year experience AVN in the ankle. Wukich believes that in terms of custom printed bones, the talus is just the tip of the iceberg.
Using 3D printing technology to create bone implants is still an emerging field, but the possibilities to reshape the field of orthopedics are nearly endless. Such implants open the door to the opportunity to correct genetic malformations or save limbs that have been damaged by trauma or disease. Procedures similar to what we are doing with talus bones are being tried for other parts of the body. For example, patients who lose a jawbone to cancer may soon be able to take advantage of this technology.
Dr. Dane Wukich, the Department of Orthopedic Surgery, UT Southwestern
References
'FDA Approves First in the World, First-of-Its-Kind Implant for the Treatment of Rare Bone Disease as a Humanitarian Use Device,' [2021], [https://www.fda.gov/news-events/press-announcements/fda-approves-first-world-first-its-kind-implant-treatment-rare-bone-disease-humanitarian-use-device]
Advanced Manufacturing for Complex Orthopaedic Procedures [https://paragon28.com/]
Dr. Dane Wukich [https://utswmed.org/doctors/dane-wukich/]
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.