Apr 7 2021
A team of researchers from the Korea Advanced Institute of Science and Technology (KAIST) has designed a bacterium that can produce carminic acid—a natural red colorant that is extensively used in cosmetics and food.
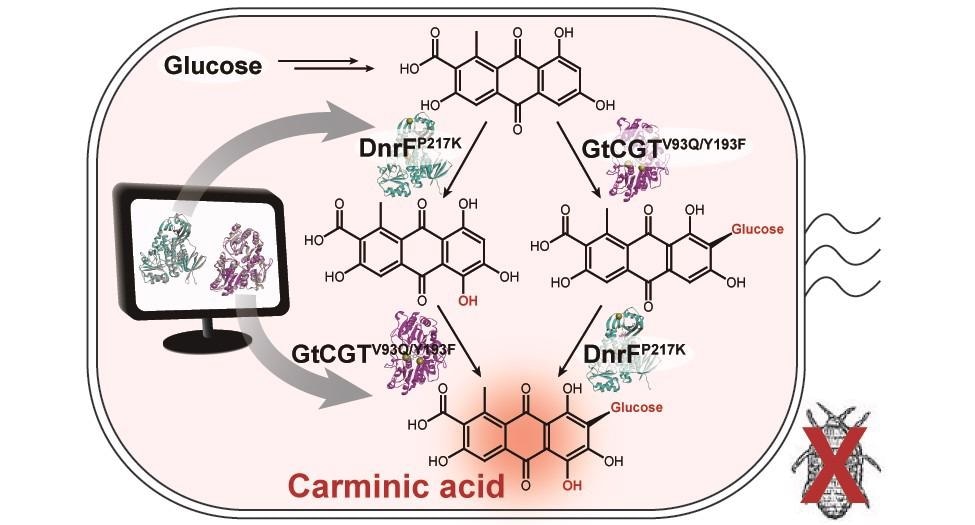 A schematic biosynthetic pathway for the production of carminic acid from glucose. Biochemical reaction analysis and computer simulation-assisted enzyme engineering were employed to identify and improve the enzymes (DnrFP217K and GtCGTV93Q/Y193F) responsible for the latter two reactions. Image Credit: The Korea Advanced Institute of Science and Technology (KAIST).
A schematic biosynthetic pathway for the production of carminic acid from glucose. Biochemical reaction analysis and computer simulation-assisted enzyme engineering were employed to identify and improve the enzymes (DnrFP217K and GtCGTV93Q/Y193F) responsible for the latter two reactions. Image Credit: The Korea Advanced Institute of Science and Technology (KAIST).
The researchers have demonstrated the entire biosynthesis of carminic acid from glucose in a newly designed Escherichia coli bacterium.
This method will prove handy for designing and constructing biosynthetic pathways that involve unfamiliar enzymes and thus the production of varied industrially significant natural products for the cosmetic, food and pharmaceutical sectors.
Being a natural red colorant, carminic acid is extensively used in many products, including lipstick and strawberry milk. But so far, carminic acid has been created by farming cochineals—a scale insect that grows only in the area around the Canary Islands and Peru, followed by complex multi-step purification procedures.
Most often, carminic acid also contains protein contaminants that lead to allergies and the majority of consumers prefer not to eat products based on insect-derived colorants.
Keeping this aspect in mind, manufacturers worldwide are utilizing alternative red colorants, irrespective of the fact that carminic acid is one among the most stable natural red colorants.
Such challenges motivated the metabolic engineering research team from KAIST to deal with this problem. Members of this research group include postdoctoral researchers Dongsoo Yang and Woo Dae Jang, and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering.
The new study titled “Production of carminic acid by metabolically engineered Escherichia coli” was published online in the Journal of the American Chemical Society (JACS) on April 2nd, 2021.
For the first time, the study has reported the development of a bacterial strain that can produce carminic acid from glucose through metabolic engineering as well as computer simulation-assisted enzyme engineering.
The team improved the type II polyketide synthase machinery to successfully create the carminic acid precursor, called flavokermesic acid.
Since the enzymes that account for the remaining two reactions were neither functional nor discovered, the researchers performed biochemical reaction analysis to detect enzymes that are capable of converting flavokermesic acid into carminic acid.
The researchers subsequently performed homology modeling and docking simulations to improve the activities of the two detected enzymes. They were able to demonstrate that carminic acid could be directly produced from glucose by the final engineered strain.
It was found that the C-glucosyltransferase designed in this analysis is typically relevant to other natural products as demonstrated by the effective production of a supplementary product—aloesin—which is present in aloe leaves.
The most important part of this research is that unknown enzymes for the production of target natural products were identified and improved by biochemical reaction analyses and computer simulation-assisted enzyme engineering.
Dongsoo Yang, Postdoctoral Researcher, KAIST
Yang explained that the development of a commonly relevant C-glucosyltransferase is also handy because C-glucosylation is a comparatively unexplored reaction in bacteria, such as Escherichia coli. The researchers used the C-glucosyltransferase engineered in this research work to effectively produce both aloesin and carminic acid from glucose.
A sustainable and insect-free method of producing carminic acid was achieved for the first time in this study. Unknown or inefficient enzymes have always been a major problem in natural product biosynthesis, and here we suggest one effective solution for solving this problem.
Sang Yup Lee, Distinguished Professor, KAIST
Professor Lee continued, “As maintaining good health in the aging society is becoming increasingly important, we expect that the technology and strategies developed here will play pivotal roles in producing other valuable natural products of medical or nutritional importance.”
The study was funded by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries of the Ministry of Science and ICT (MSIT) via the National Research Foundation (NRF) of Korea and the KAIST Cross-Generation Collaborative Lab project.
Sang Yup Lee and Dongsoo Yang were also financially supported by Novo Nordisk Foundation in Denmark.
Journal Reference:
Yang, D., et al. (2021) Production of carminic acid by metabolically engineered Escherichia coli. Journal of the American Chemical Society. doi.org.10.1021/jacs.0c12406.