May 6 2021
3D bioprinting has the ability to make engineered scaffolds that imitate natural tissue. Regulating the cellular organization inside those engineered scaffolds for regenerative applications is a complicated and tough process.
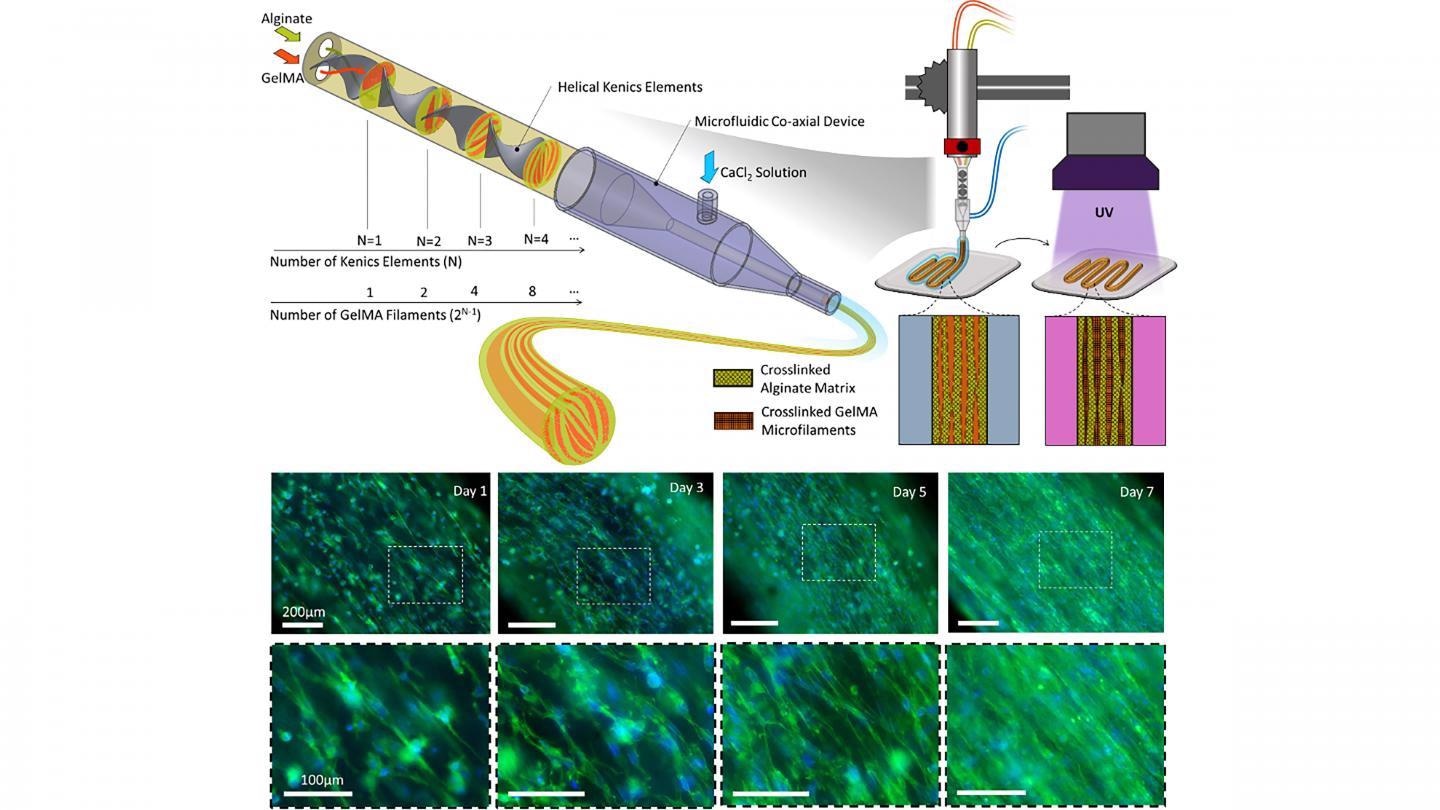 Biofabrication of multicompartmental hydrogel fibers for the formation of multiscale biomimetic constructs. Image Credit: Mohamadmahdi Samandari, Fatemeh Alipanah, Keivan Majidzadeh-A, Mario M. Alvarez, Grissel Trujillo-de Santiago, and Ali Tamayol.
Biofabrication of multicompartmental hydrogel fibers for the formation of multiscale biomimetic constructs. Image Credit: Mohamadmahdi Samandari, Fatemeh Alipanah, Keivan Majidzadeh-A, Mario M. Alvarez, Grissel Trujillo-de Santiago, and Ali Tamayol.
Cell tissues have the tendency to be highly ordered with regards to spatial distribution and alignment, hence bioengineered cellular scaffolds for tissue engineering applications should closely match this orientation to be able to work similar to natural tissue.
In the journal Applied Physics Reviews, from AIP Publishing, an international research group explains its method for guiding cell orientation inside deposited hydrogel fibers through a technique known as multicompartmental bioprinting.
The researchers make use of static mixing to fabricate striated hydrogel fibers that have been developed from packed microfilaments of various hydrogels.
In this structure, few compartments offer a fortunate surrounding for cell proliferation, while others serve as morphological cues guiding cell alignment.
The millimeter-scale printed fiber with the microscale topology has the ability to quickly arrange the cells toward quicker maturation of the engineered tissue.
This strategy works on two principles. The formation of topographies is based on the design of fluid within nozzles and controlled mixing of two separate precursors. After crosslinking, the interfaces of the two materials serve as 3D surfaces to provide topographical cues to cells encapsulated within the cell permissive compartment.
Ali Tamayol, Study Co-Author and Associate Professor in Biological Engineering, UConn Health
Extrusion-based bioprinting is the most extensively utilized bioprinting technique. In extrusion-based bioprinting, the printed fibers are normally several hundreds of micrometers in size with randomly oriented cells, so a method offering topographical cues to the cells inside such fibers to guide their organization is very convenient.
Traditional extrusion bioprinting experiences high shear stress applied to the cells at the time of the extrusion of fine filaments. However, the fine scale features of the suggested method are passive and do not compromise other parameters included in the printing process.
The team feels that for the direct cellular organization to be performed, extrusion-based 3D-bioprinted scaffolds must be made from very fine filaments.
It makes the process challenging and limits its biocompatibility and the number of materials that can be used, but with this strategy larger filaments can still direct cellular organization.
Ali Tamayol, Study Co-Author and Associate Professor in Biological Engineering, UConn Health
This bioprinting method “enables production of tissue structures' morphological features —with a resolution up to sizes comparable to the cells' dimension—to control cellular behavior and form biomimetic structures. And it shows great potential for engineering fibrillar tissues such as skeletal muscles, tendons, and ligaments,” concluded Tamayol.
Journal Reference:
Samandari, M., et al. (2021) Controlling cellular organization in bioprinting through designed 3D microcompartmentalization. Applied Physics Reviews. doi.org/10.1063/5.0040732.