May 13 2021
Smart materials are a new revolution in the area of materials science and can acclimatize their properties based on their environmental changes.
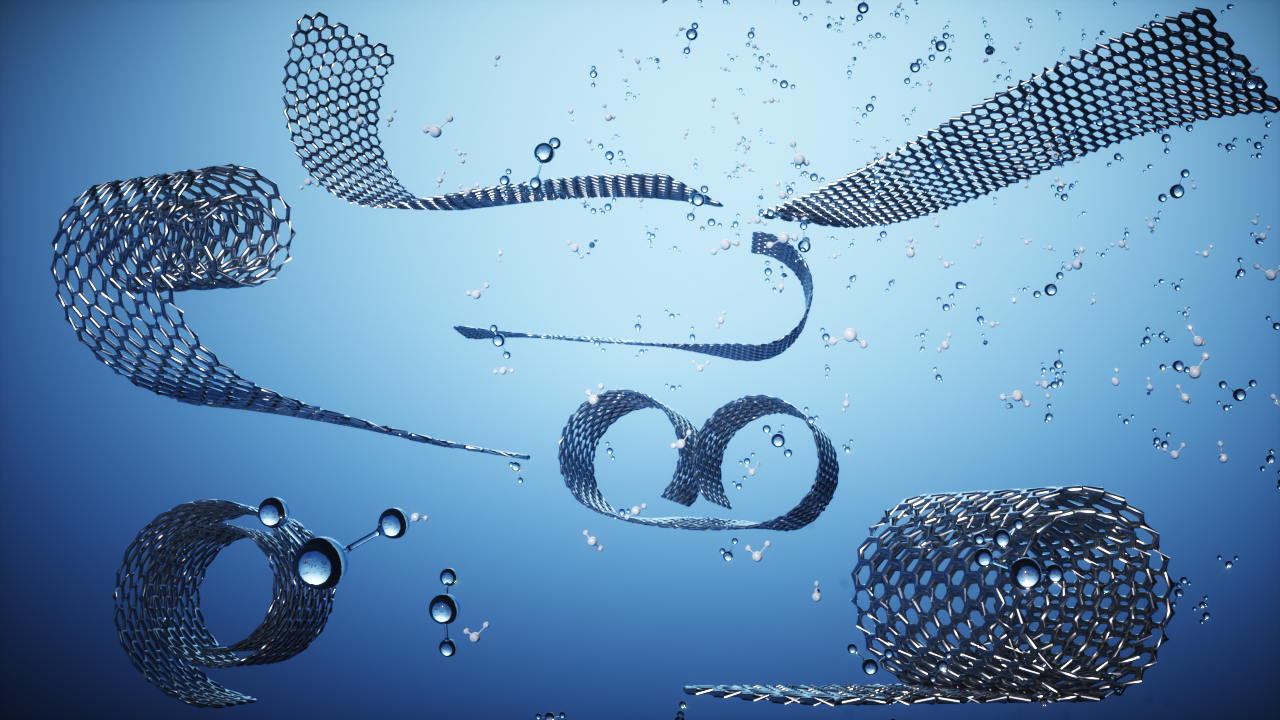 By altering the pH of the suspensions, the NUS researchers demonstrated the ability of the 2D-electrolyte sheet to roll up into a scroll-like arrangement. This is similar to the way electrically charged polymers undergo transitions from molecular chains to globular objects. Image Credit: National University of Singapore.
By altering the pH of the suspensions, the NUS researchers demonstrated the ability of the 2D-electrolyte sheet to roll up into a scroll-like arrangement. This is similar to the way electrically charged polymers undergo transitions from molecular chains to globular objects. Image Credit: National University of Singapore.
Smart materials can be used in many different things, such as targeted drug delivery, shape-shifting airplane wings, and self-healing mobile phone screens. Using smart materials to deliver drugs to a specific target within the body is specifically significant for various diseases, including cancer. This is because such materials only discharge the drug payload upon detecting the presence of a tumor cell and do not harm the healthy cells.
Scientists from the Centre for Advanced 2D Materials (CA2DM) at the National University of Singapore (NUS) have now designed a new class of smart materials that have the structure of a 2D material but act similar to an electrolyte. These materials could provide a new way to deliver drugs inside the body.
The new “2D-electrolytes,” similar to conventional electrolytes, dissociate their atoms in various solvents and eventually become electrically charged. Moreover, the organization of these smart materials can be regulated by external factors, like temperatures and pH, which are perfect for targeted drug delivery. The 2D-electrolytes also hold potential for other applications that need a material to be responsive to changes in the environment, like energy storage and artificial muscles.
The researchers behind the 2D-electrolytes were headed by Professor Antonio Castro Neto, Director of CA2DM, and included scientists from CA2DM, the NUS Department of Physics, and the NUS Department of Materials Science and Engineering.
The team’s breakthrough findings were published in the leading Advanced Materials journal on 12th May 2021.
Changing the Behavior of 2D Materials
In the field of materials science, a 2D material is a solid material existing in one layer of atoms. This material can be considered as an atomically thin sheet that has a particular width and height, but effectively no depth, and therefore, it is fundamentally two-dimensional. By contrast, an electrolyte is a substance that creates an electrically conducting suspension when dissolved in a solvent, like water.
Many 2D materials exist today and the behavior of electrolytes has been proven in an unlimited number of other compounds. But the results from the NUS team demonstrate the first example of materials that exhibit both the properties and 2D structure of electrolytes, with a specific tendency to shapeshift their form reversibly in a liquid medium.
To achieve this feat, the NUS researchers used organic molecules as reactive species to introduce different functionalities to 2D materials, like molybdenum disulfide (MoS2) and graphene.
By adding different chemical groups that become positively or negatively electrically charged in solvents, we altered traditional 2D materials and came up with a novel class of smart materials that have their electronic properties controlled by morphological conformation.
Antonio Castro Neto, Professor and Director, Centre for Advanced 2D Materials, National University of Singapore
The techniques used by the team to produce 2D-electrolytes are just a few potential examples among many promising options, rendering this discovery a new, fascinating research field to investigate.
From a Flat Sheet to a Rolled-up Scroll
A crucial advancement of this study was that the orientation of the 2D-electrolytes can change reversibly by adjusting the external conditions. At present, the electrical repulsion that exists between the surface charge in a 2D material allows it to be placed on a flat sheet.
By changing the ionic concentration, temperature, and pH of the suspensions, the NUS team revealed the potential of the 2D-electrolyte sheet to shapeshift and create scroll-like arrangements. Such experimental results are supported by comprehensive theoretical analysis where they explain the physical mechanism involved in the formation and stability of scrolls orientations.
Such scroll orientations have a relatively small diameter and hence can be described as one-dimensional (1D), resulting in different chemical and physical characteristics. This conversion from 2D to 1D can also be reversed by restoring the external conditions to their original values.
One can think of 2D-electrolytes as the higher dimensional analogues of 1D electrolytes, commonly known as polyelectrolytes.
Antonio Castro Neto, Professor and Director, Centre for Advanced 2D Materials, National University of Singapore
Major examples of polyelectrolytes comprise several biologically relevant materials, like RNA and DNA.
“When acids, bases, or salts are added, these electrically charged polymers also undergo conformational transitions from molecular chains that are 1D, to globular objects of 0D, and vice versa. Our 2D-electrolytes, in analogy with polyelectrolytes, show reversible transitions from 2D to 1D, as a function of external factors. As stimuli-responsive materials, they are suitable for the creation of cutting-edge technology,” added Professor Castro Neto.
Next Steps
Identifying this group of materials has paved the way to new fields of exploration for materials scientists, as it brings together two areas of studies, such as electrolytes in the field of Electrochemistry and 2D materials in the area of Physics, that have been conventionally unrelated.
There is an uncountable number of ways to functionalise graphene and other 2D materials to transform them into 2D-electrolytes. We hope that our work will inspire scientists from different fields to further explore the properties and possible applications of 2D-electrolytes.
Antonio Castro Neto, Professor and Director, Centre for Advanced 2D Materials, National University of Singapore
“We anticipate that as 2D-electrolytes have similarities with biological or natural systems, they are capable of spontaneously self-assemble and cross-link to form nanofibers that are promising for applications in filtration membranes, drug delivery, and smart e-textiles,” concluded Professor Castro Neto.
Journal Reference:
Costa, M. C. F., et al. (2021) 2D Electrolytes: Theory, Modeling, Synthesis, and Characterization. Advanced Materials. doi.org/10.1002/adma.202100442.