May 18 2021
In the ongoing pursuit to change into a more environmentally friendly society, hydrogen (H2) is hailed as the clean fuel of the future.
 Scientists find an optimal hydrogen-natural gas blend to trap hydrogen in cage-like molecules more effectively. Image Credit: Gwangju Institute of Science and Technology (GIST).
Scientists find an optimal hydrogen-natural gas blend to trap hydrogen in cage-like molecules more effectively. Image Credit: Gwangju Institute of Science and Technology (GIST).
Since H2 can be generated from water (H2O) without producing carbon emissions, it has become a top priority to design H2-compatible technologies. But the road ahead is not easy, and several technical restrictions must be overcome.
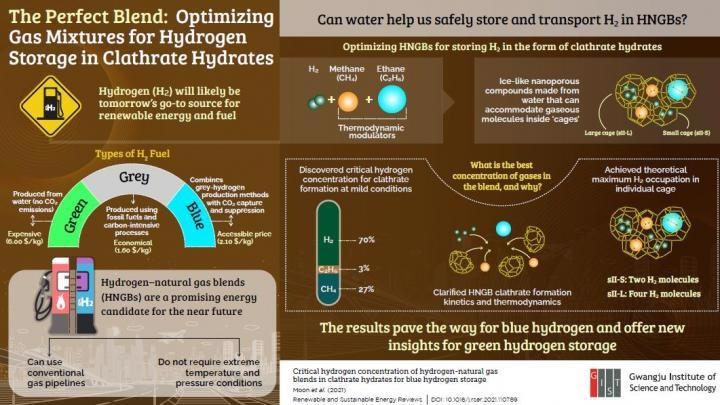
Hydrogen is the smallest molecule in nature and finding feasible ways to store it is a critical issue to realize a hydrogen economy.
Youngjune Park, Associate Professor, Gwangju Institute of Science and Technology
Pure H2 is different from hydrocarbons and should be preserved at low temperatures (20 °C). or very high pressures (more than 100 atmospheres). This naturally signifies a huge economic obstacle for storing H2. But what if H2 is confined within ice-like crystals to ease transportation and storage?
Molecular cages like these occur in nature and are referred to as “clathrate hydrates.” These cages are solid water-based compounds that have cavities to accommodate numerous molecules.
Dr Park’s team from the Gwangju Institute of Science and Technology (GIST) has been studying the application of clathrate hydrates as vessels for storing H2. But the enclathration of pure H2 continues to be a gradual process that also demands high-pressure and high-temperature conditions.
In a new study, recently reported in volume 141 May 2021 print issue of the Renewable and Sustainable Energy Reviews journal, Dr Park’s team investigated a viable solution to address this issue.
Previous scientists did not attempt to create clathrate hydrates from pure H2 but rather recommended mixing it with natural gas, which was experimentally demonstrated to support enclathration at more subtle conditions.
To further enhance this method, the GIST team set out to identify the most optimal hydrogen-natural gas blend (HNGB) to form clathrate hydrates in an energy-efficient manner.
As such, the researchers thoroughly studied the clathrate hydrates created from HNGBs with varying concentrations of hydrogen, ethane, and methane. Then, they carefully examined the kinetics of clathrate formation and the distribution and structure of the trapped molecules.
The researchers were able to detect the accurate concentrations of gas at which point ethane and methane, serving as thermodynamic modulators, optimally improve the H2 storage capacity of HNGB hydrates.
Even at moderate temperature and pressure conditions (less than 8 °C and less than 100 atmospheres, respectively), the researchers attained the highest theoretical H2 storage possible for two kinds of clathrate hydrate cages—four and two H2 molecules in large and small cages, respectively. This breakthrough had not been reported in the past and the unparalleled study results could thus aid the development of HNGB hydrate storage media.
Clathrate hydrates and HNGBs could provide a reasonable mid-term solution for storing what is known as ‘blue’ hydrogen, which is hydrogen produced using fossil fuel-based technology but with minimal CO2 emissions.
Youngjune Park, Associate Professor, Gwangju Institute of Science and Technology
At present, blue hydrogen is three times more economical to produce when compared to environmentally friendly “green” hydrogen. Hence, the study results may help ease the slow transition from fossil fuels toward hydrogen, which is crucial to a sustainable future.
Journal Reference:
Moon, S., et al. (2021) Critical hydrogen concentration of hydrogen-natural gas blends in clathrate hydrates for blue hydrogen storage. Renewable and Sustainable Energy Reviews. doi.org/10.1016/j.rser.2021.110789.