A biodegradable and customizable 3D printed vascular graft could help prevent heart failure in cardiovascular patients.
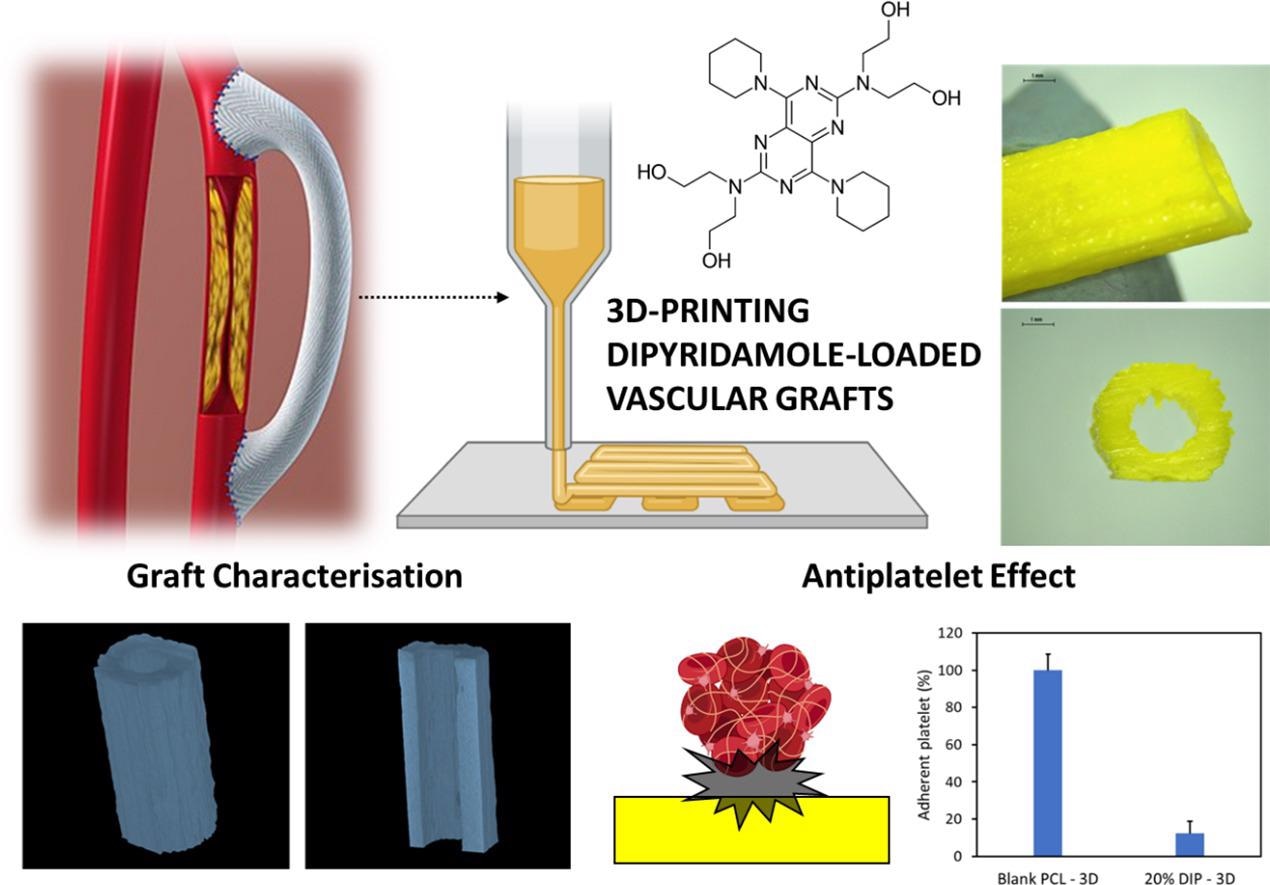 Image Credit: Juan Domínguez-Robles, Tingjun Shen, Victoria A. Cornelius, et al, [2021], ‘Development of drug-loaded cardiovascular prosthesis for thrombosis prevention using 3D printing,’ Materials Science and Engineering: C, [https:]//doi.org/10.1016/j.msec.2021.112375.
Image Credit: Juan Domínguez-Robles, Tingjun Shen, Victoria A. Cornelius, et al, [2021], ‘Development of drug-loaded cardiovascular prosthesis for thrombosis prevention using 3D printing,’ Materials Science and Engineering: C, [https:]//doi.org/10.1016/j.msec.2021.112375.
Cardiovascular disease (CVD) — a general term for a range of conditions affecting the heart and blood vessels, including heart failure, arterial disease, and stroke — is the leading cause of death globally, accounting for the loss of almost 18 million people in 2016 alone. By 2030 the cost of CVDs in terms of mortality is expected to rise to around 24 million deaths worldwide.
Thus tackling CVD efficiently is a major concern for health care providers, with a key factor in preventing heart failure during these events being the quick restoration of blood flow to the heart.
Currently, the best way of doing this is revascularization by either placing a stent or by performing a surgical bypass. This latter technique hinges on the use of autologous vessels to bypass diseased or blocked arteries. But, the procedure is not without risk. Harvesting vessels for use in this procedure is invasive and not without risk. Even after successful harvesting, the possibility remains that the vessels will be usable.
There is a possible alternative, however. Researchers are currently hard at work developing synthetic vascular grafts. For maximum effectiveness, these grafts should be flexible with adjustable size and length, be ready for use in a practical setting, and crucially made from biodegradable materials.
Current polymers used for artificial grafts have exceptional biocompatibility, chemical stability with low toxicity, and robustness that makes them a long-term solution. Unfortunately, as well as lacking that crucial biodegradability, these materials have thus far proven to have limited success when used to replace smaller blood vessels.
One solution to these issues is the creation of grafts that can be embedded with antithrombotic drugs. Alternatively, materials could be combined to create a graft that gradually degrades and is replaced by natural blood vessels.
A new paper¹ published in the journal Materials Science and Engineering: C suggests a biodegradable, non-toxic, artificial vascular graft made from polycaprolactone (PCL) that has the potential to degrade gradually and thus allow the regeneration of blood vessels. The material has already been approved for use as a biomaterial in implants and for drug delivery.
The authors of the study aimed to put 3D printed PCL-based vascular grafts loaded with dipyridamole (DIP) — a widely used antithrombotic drug — to the test.
Testing Biodegradable PCL-based Vascular Grafts
The researchers tested two different forms of PCL, which varied in molecular weight, combining the material with three different concentrations of DIP — 5%, 10% and 20%. The PCL-based grafts were designed using computer-aided design (CAD) software and were then printed using a 3D bioprinter — a Bioscaffolder 3.2, GeSiM².
The researchers also evaluated the morphology of the grafts and their thermal properties using scanning electronic microscopy (SEM) and by subjecting the material to temperatures of around 60⁰C. Following this, the term conducting mechanical testing upon the grafts finding the material’s peak strengths and the point at which strain causes mechanical failure. SEM was also used to find how well the PCL and DIP combined via hydrogen bonding.
The researchers discovered that while the substances successfully mixed without the development of DIP crystals, the surfaces of the grafts became rough after the percentage of DIP was increased. The team also found that lower molecular weight PCL allowed the DIP to be more successfully integrated. This factor could be used in actual grafts to do away with the need for solvents which can potentially pose a risk to patients.
Perhaps most significantly, the team found that 3D printing can be used for the production of small vascular grafts, especially for grafts not ‘doped’ with DIP. Though not as well suited for smaller arteries, the grafts that were infused with DIP were found to be capable of sustained and controlled drug release for at least 30 days without any sudden large releases or ‘bursts’ of the antithrombotic.
The team does point out that many aspects of the PCL-based vascular grafts will need to be further evaluated before clinical use. This will include a thorough investigation of how 3D printers for in-situ clinical use can be effectively sterilized.
The concern expressed by the team is that some current sterilizing methods — including gamma irradiation and the use of ethylene oxide — could alter the properties of PCL-based vascular grafts.
Despite these concerns and the need for in vitro testing, the PCL grafts seemed to perform well and could soon provide an artificial, biodegradable, and personalized intervention for CVD patients.
“The obtained results suggest that 3D printing can be successfully used for this purpose, and therefore has a great potential to be transferred to clinical applications,” say the authors. “Moreover, this emerging technology allows the manufacture of medical devices on-demand, with modifications in the shape, size, and dose, allowing the devices to be personalized/designed to the individual needs of each patient.”
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
1. Juan Domínguez-Robles, Tingjun Shen, Victoria A. Cornelius, et al, [2021], ‘Development of drug-loaded cardiovascular prosthesis for thrombosis prevention using 3D printing,’
Materials Science and Engineering: C, [https:]//doi.org/10.1016/j.msec.2021.112375.