Climate change has made the development of green, clean energy solutions a global imperative. Solar energy is already a widely accepted technology and offers a renewable, clean source of energy that can be fed back into the electricity grid or stored. Now, a recent collaborative study, published in the journal Cell Reports Physical Science, has been presented which demonstrates how using mesoporous carbon could potentially improve the stability and performance of perovskite photovoltaic cells.

Study: Light-induced performance increase of carbon-based perovskite solar module for 20-year stability. Image Credit: aleks333/Shutterstock.com
However, one of the limitations of solar technology is often related to the stability and longevity of the materials used in the manufacture of perovskite solar cells. Therefore, improving upon the material stability of perovskite photovoltaics has become one of the main objectives in practical application.
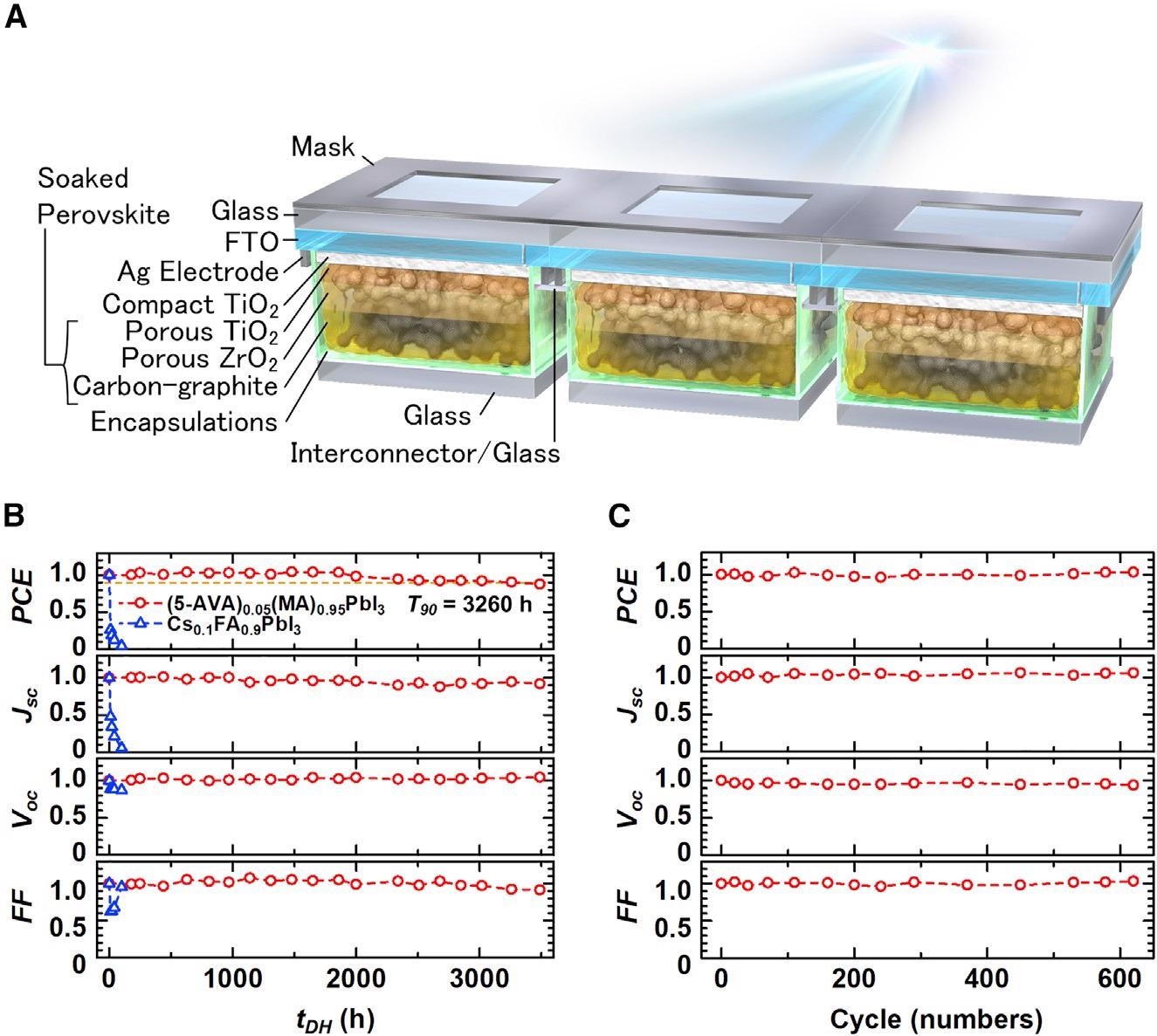
m-CPSM structure and results of stability tests. (A) Schematic of m-CPSM structure: 3-cell module, aperture area of 1.92 cm2. (B) Damp-heat (85 °C/85% RH) results of (5-AVA)0.05(MA)0.95PbI3 and Cs0.1FA0.9PbI3. (C) Thermal cycling (-40 °C to 85 °C) results of (5-AVA)0.05(MA)0.95PbI3. (B and C) Each value is normalized by its initial value. Image Credit: Eiji Kobayashi et al., Cell Reports Physical Science
A team of researchers has claimed mesoporous carbon could potentially improve the stability and performance of perovskite photovoltaic cells; the encapsulated mesoporous carbon solar mini-modules retained more than 92% of their initial performance after 3000 hours of damp-heat aging which is equivalent to 20-year stability in practical outdoor use.
Light-Soaking Treatment
Typically, the lack of stability under prolonged light soaking (LS) is one of the intrinsic characteristics of perovskite cells which holds the technology back somewhat. One phenomenon associated with light stability is light-induced degradation (LID), also known as metastability.
However, the cells in this study were able to demonstrate such outstanding performance attributes and stability due to a reversible light-induced phenomenon which was provoked by a reaction between methylammonium – a key material of the perovskite layer – and 5-ammoniumvaleric acid.
“This stability is attributed to the light-induced performance increase phenomenon. The mechanism is associated with the organic molecules 5-ammoniumvaleric acid and methylammonium forming a quasi-2-dimensional perovskite/metal oxide interface with a positive effect on charge transport and ion migration,” explains Dr. Eiji Kobayashi, lead author and researcher at Kishu Giken Kogyo Co,. Ltd.
Consequently, the positive effect on charge transport and ion migration increased the efficiency absolute gain up to 1.4% (16% relative) within 10 minutes and retained the relative stability for around 100 hours. One of the standout findings was this effect exhibited “reversibly improved” performance with a return to initial efficiency when stored in the dark and increasing again in low-light conditions.
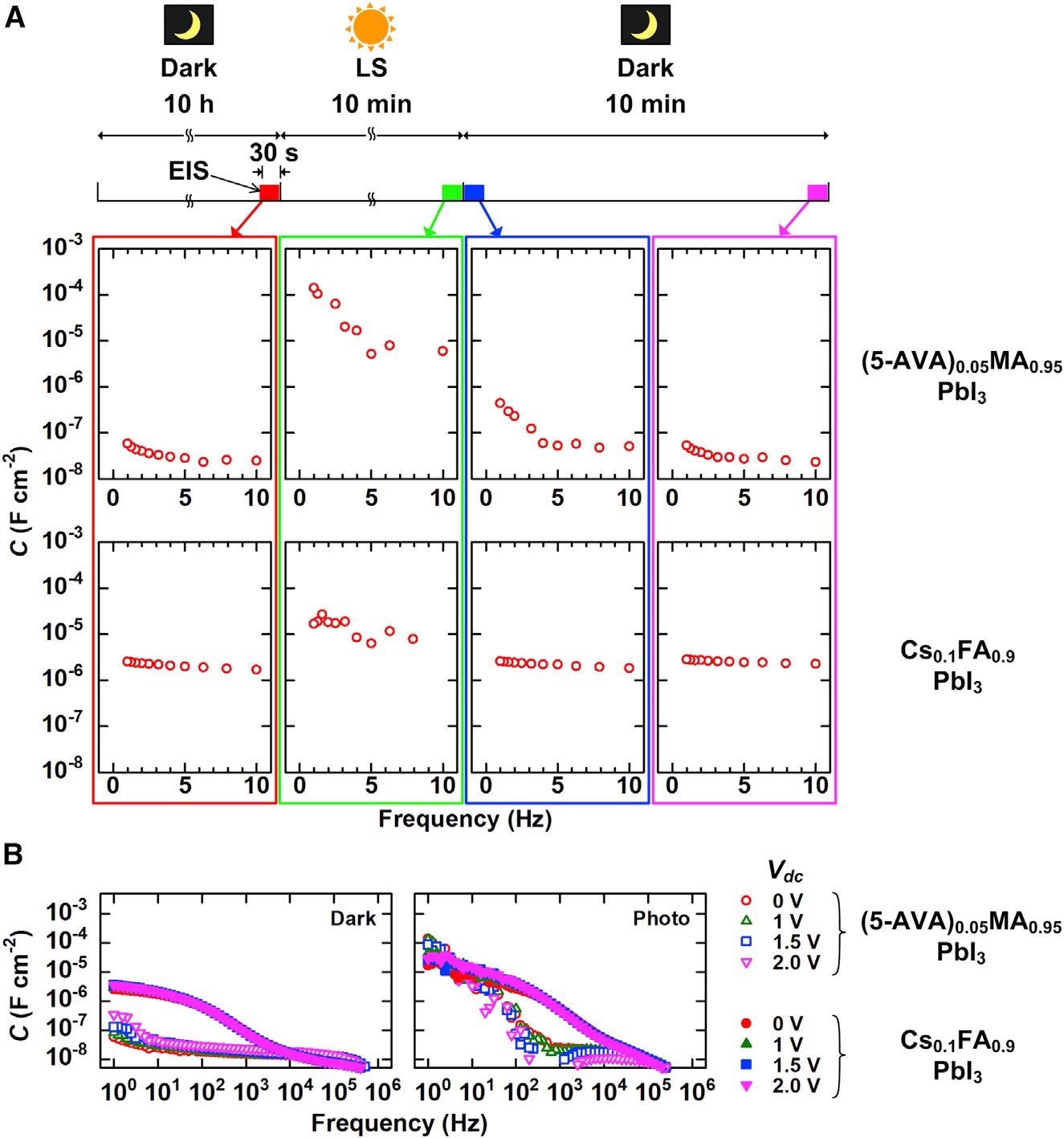
Apparent capacitance (C = Im(Z-1)ω-1) obtained from EIS data for m-CPSM with (5-AVA)0.05MA0.95PbI3 and Cs0.1FA0.9PbI3 (A) Evolution of C values during cycles of storage in darkness for 10 h, 1-sun LS for 10 min, and storage in darkness again for 10 min at low frequency (≤10 Hz). (B) C values for wide frequency region (106 to 106 Hz) with Vdc = 0, 1, 1.5, and 2 V. Dark capacitance (left) and photo capacitance (right). Image Credit: Eiji Kobayashi et al., Cell Reports Physical Science
“Our investigation reveals that this phenomenon is linked to the built-in electric field created either by LS or device biasing and the content of MA cation and 5-ammoniumvaleric acid iodide (5-AVAI) additives,” said Kobayashi.
Perovskite Cell Fabrication
Perovskite is used to describe any material or chemical compound possessing a crystal structure similar to the naturally occurring mineral also called perovskite (a mineral of calcium titanium oxide) first discovered in Russia’s Ural Mountains in 1839 by Gustav Rose and named after Russian mineralogist Lev Perovski.
The mesoporous carbon perovskite solar cell (m-CPSM) devices Kobayashi and his colleagues fabricated employed commercial-grade substrates and monolithic electrodes: this included titanium-oxide (TiO2) and zirconium dioxide (ZrO2) layers.
These layers were then set with a thermoplastic resin using an encapsulation process and then deposited on fluorine-doped tin oxide glass. One of the key findings of the stability tests was that as a result of the novel encapsulation process, the technology was successful at holding back degradation associated with oxygen and moisture: “This marks the highest stability for perovskite solar devices to the best of our knowledge,” Kobayashi stated.
Furthermore, the LS tests – which were conducted using a commercial light source – revealed that the LS effect can be activated even by indoor light with an illuminance as low as 200 lux. The LS effects were closely associated with the intrinsic electric field resulting from either LS or device biasing, in addition to the MA+ and 5-AVA+contents of the perovskite.
Thus, the LS treatment and technique described by the researchers can facilitate accurate evaluation, stable operation, and increased performance all of which boost the practical applications of perovskite photovoltaics.
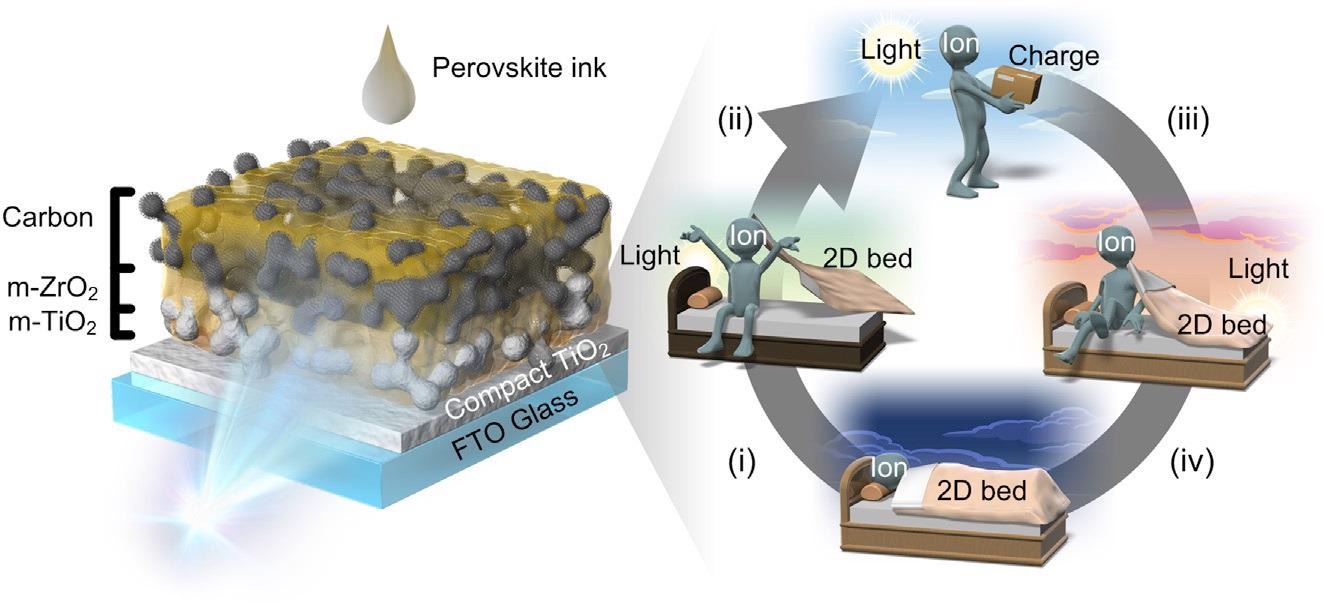
Schematics of a reversible light-induced performance increase for m-CPSM Considerable evolution ofMA+ ionmigration at interface between TiO2 nanoparticles (m-TiO2) and quasi-2D perovskite during LS is shown: (i) Ion migration is activated by light absorption in quasi-2D perovskite. Essentially, a perfect perovskite crystal does not cause ion migration, but perovskite crystals in mesoporous electrodes have many defects and are prone to ion migration. Although the presence of quasi-2D perovskite crystals on the surface of m-TiO2 makes it difficult for ion migration, the quasi-2D crystals are disentangled during LS, possibly leading to ion migration. (ii) Light-assisted ion-migration contributes to interfacial charge accumulation, with the result that the performance increases due to easy electron transport. (iii) Migrated ions return to quasi-2D perovskite during storage under dark conditions. (iv) Performance reverts to low initial value. Image Credit: Eiji Kobayashi et al., Cell Reports Physical Science
Additionally, Kobayashi and his team used industry-compatible processes and materials and they expect the light soaking treatment could be equally effective for large-scale commercial devices.
As current, mainstream solar technology (primarily silicon-based PVs) reaches practical and economic PV efficiency limits, making improvements to perovskite cells will allow solar cell manufacturers to advance far beyond any limiting performance barriers.
Furthermore, such advances could transform the solar-cell market and accelerate the growth and wide-scale production of perovskite solar cells. This is of particular relevance as the carbon-emissions clock is now winding down to 2050 after the recent COP26 summit so getting perovskite out of the lab and into scalable production could revolutionize the clean energy industry.
References
Eiji Kobayashi, Ryuki Tsuji, David Martineau, Andreas Hinsch, Seigo Ito, ‘Light-induced performance increase of carbon-based perovskite solar module for 20-year stability’, Cell Reports Physical Science. 2021. https://www.sciencedirect.com/science/article/pii/S2666386421003702?via%3Dihub
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.