In an article recently published in the open-access journal Applied Surface Science Advances, researchers from the University of Sharjah, UAE, fabricated low-cost and recyclable electrodes by depositing iron and copper alloy on an aluminum sheet. The resulting electrode demonstrated a 20-40% increase in transmission in aqueous solutions.
Study: Utilizing aluminum sheets with FeCu deposits as cheap water cleaning electrodes. Image Credit: joserpizarro/Shutterstock.com
Cheap and Recyclable Devices for Iron Detection and Removal from Water?
Iron contamination above the permissible limit in potable water resources such as rivers and aquifers is hazardous to human health and the surrounding ecosystem. There are already devices that can sense the iron content in water and remove them effectively.
These consist of a substrate that hosts an active or sensitive layer that can be deployed within target media. The active layer has the desired affinity in targeting an analyte or contaminant and drawing it to the surface of the sensing element.
There are several methods to deposit this kind of sensitive film on the electrode surface viz. chemical vapor deposition (CVD), physical deposition (sputtering), or mechanical process. The latter is highly preferable due to their reliability, cost-effectiveness, and being dry technologies that do not require chemical additives or specialized operating conditions such as temperature or pressure.
![XRD results of the development of the single-phase Fe-Cu [22].](https://www.azom.com/images/news/ImageForNews_57494_16381892761227056.jpg)
XRD results of the development of the single-phase Fe-Cu. Image Credit: Hawili, A., Applied Surface Science Advances
Planetary ball mills work on the principle of introducing specimens to centrifugal forces around two or more axes. This technique is commonly used to synthesize alloys that are naturally immiscible or difficult to produce through conventional alloying techniques.
It utilizes the kinetic energy of the moving steel balls rather than supplying thermal energy or pressure on the alloy composition. This method is highly efficient over other techniques used for coating surfaces with magnetic materials such as electroplating and laser-assisted heating.
About the Study
The researchers coated Fe-Cu metastable alloy layer with the ferromagnetic property on a cantilever beam-shaped aluminum substrate. The alloy possesses outstanding mechanical, electrical, thermal, and magnetic properties.
The coating method relies on a simple sustainable technique that utilizes the kinetic energy of the Fe and Cu particles moving inside a planetary ball mill. The amount of ultraviolet and visible light transmitted by the solution consisting of Fe powder and deionized water is measured using a spectrometer, and an aluminum substrate coated with Fe50Cu50 is immersed in the solution for a few seconds to observe the changes in transmission and compare with that of the previous state.
This experimental methodology involves three major steps namely, synthesis and deposition of sensing material on the substrate, measurement of magnetic and electrical transport properties, and conducting optical property measurements to assess the efficacy of iron removal.
The new Fe50Cu50 solid-state solution is prepared via mechanical alloying of the precursors of iron and copper powders in a planetary ball mill. The coating part of the aluminum substrate takes place within the same grinding bowls but after removing the steel balls.
The substrate in the shape of the collar is fixed on the inner periphery of the bowls while the ball milling machine is operated at high speeds. The centrifugal kinetic energy of the small particles inside is sufficient to form Fe50Cu50 solid solution, which later gets deposited on the substrate of choice.
![(a) Field Emission-SEM images of the Fe-Cu powder (b) TEM of FeCu material showing a small cluster of FeCu [25].](https://www.azom.com/images/news/ImageForNews_57494_16381892867834917.jpg)
(a) Field Emission-SEM images of the Fe-Cu powder (b) TEM of FeCu material showing a small cluster of FeCu. Image Credit: Hawili, A., Applied Surface Science Advances
Observations
The XRD and SEM analyses revealed a single face-centered cubic (FCC) phase uniform coating. Also, these particles tend to cluster due to their strong cohesive forces. It is found that the coating thicknesses of the samples coated for 5 hours are higher than those coated for 2 hours, although 800 rpm speed is found to be destructive to the thin aluminum substrates.
The transmission data show transmission in the visible region after the magnetized aluminum electrode is exposed to an aqueous Fe solution. The results in the UV range show many sharp peaks as the presence of iron in deionized (DI) water promotes reflection or absorption, especially around 262 nm as previously reported. This indicates the interaction of UV light with iron particles due to the collective oscillation of the surface plasmons.
In the case of fcc-FexCu100-x solid solutions, different atomic sizes of Cu and Fe will alter the distance between nearest neighbors (between Fe) significantly and may result in quite large magnetic moments for Fe in FCC environments. It is previously reported that the Fe-based alloys are very susceptible to the subtle variations in the atomic volume or interatomic Fe-Fe distance.
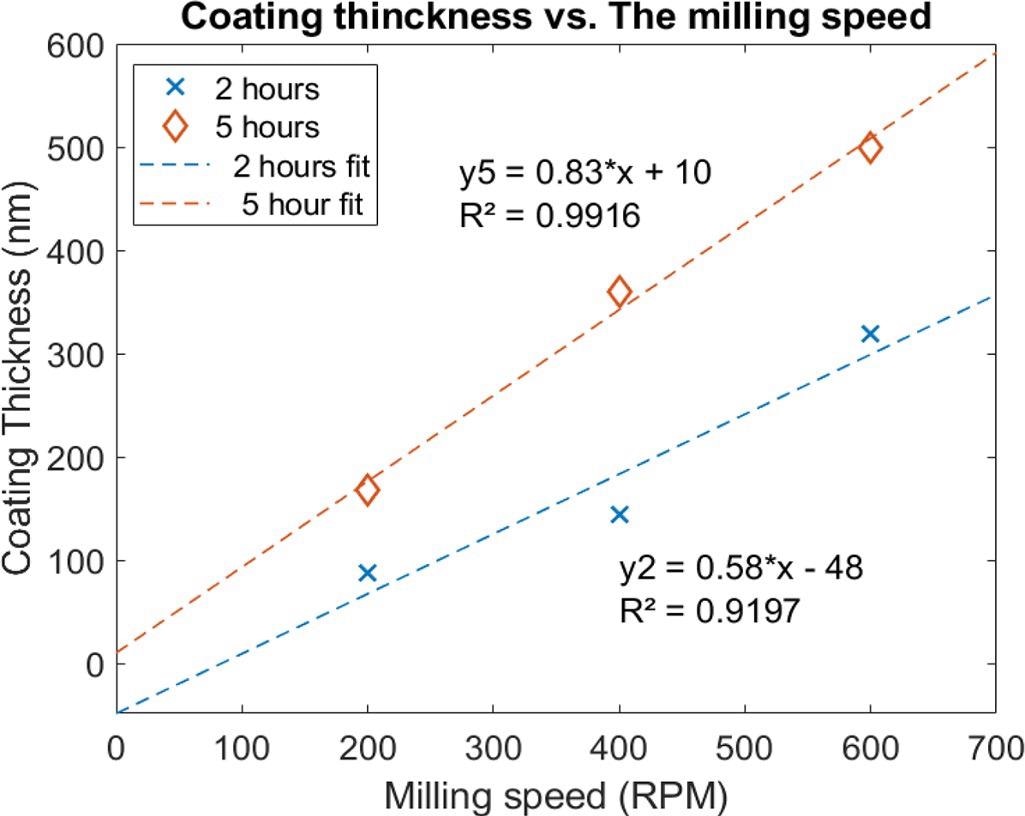
Variations of the coating thickness with the milling speed were obtained for two sets of the beam samples (coating time of 2 h and 5 h). Image Credit: Hawili, A., Applied Surface Science Advances
Conclusions
In this study, the coated beam specimens were used in an aqueous solution of fine iron powder and spectral transmission tests showed an enhancement from 20% to 40%, indicating that a considerable amount of Fe powder was removed.
The authors used materials that are available, cheap, and recyclable, where the coating can be added multiple times to the substrate or removed and applied again, and the aluminum substrate can be recycled. This technology paves way for the fabrication of electrodes that are both sustainable and cost-effective.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Hawili, A., Ghommem, M., Alami, A., Alasad, S., Egilmez, M., Zaid, W., Utilizing aluminum sheets with FeCu deposits as cheap water cleaning electrodes, Applied Surface Science Advances, Volume 7, 2022, 100193, ISSN 2666-5239, https://doi.org/10.1016/j.apsadv.2021.100193, https://www.sciencedirect.com/science/article/pii/S2666523921001392?via%3Dihub