Researchers from China developed a new anode material for potassium-ion batteries by encapsulating antimony-sulfur nanorods in reduced graphene oxide and nitrogen-doped carbon. The synergy between double physical protection and carbon-antimony chemical bonding enhanced the stability and electrochemical kinetics of the resulting anode. This study is published in the journal iScience.
Study: Sb2S3- Based Conversion-Alloying Dual Mechanism Anode for Potassium-Ion Batteries. Image Credit: Rost9/Shutterstock.com
Advantages and Limitations of Potassium-Ion Batteries
Despite the wide use of lithium-ion batteries (LiBs), the limited lithium reserves, and very few countries controlling the lithium supply chain, alternative solutions such as potassium-ion batteries (PiBs) have drawn the attention of many researchers.
PiBs are cheap, highly available, and show similar ‘rocking chair’ electrochemical behavior as LiBs. Rocking chair-type batteries operate based on two reversible electrochemical redox processes separately occurring at anode and cathode, and the same ion is intercalated or deintercalated at both electrodes during charging or discharging.
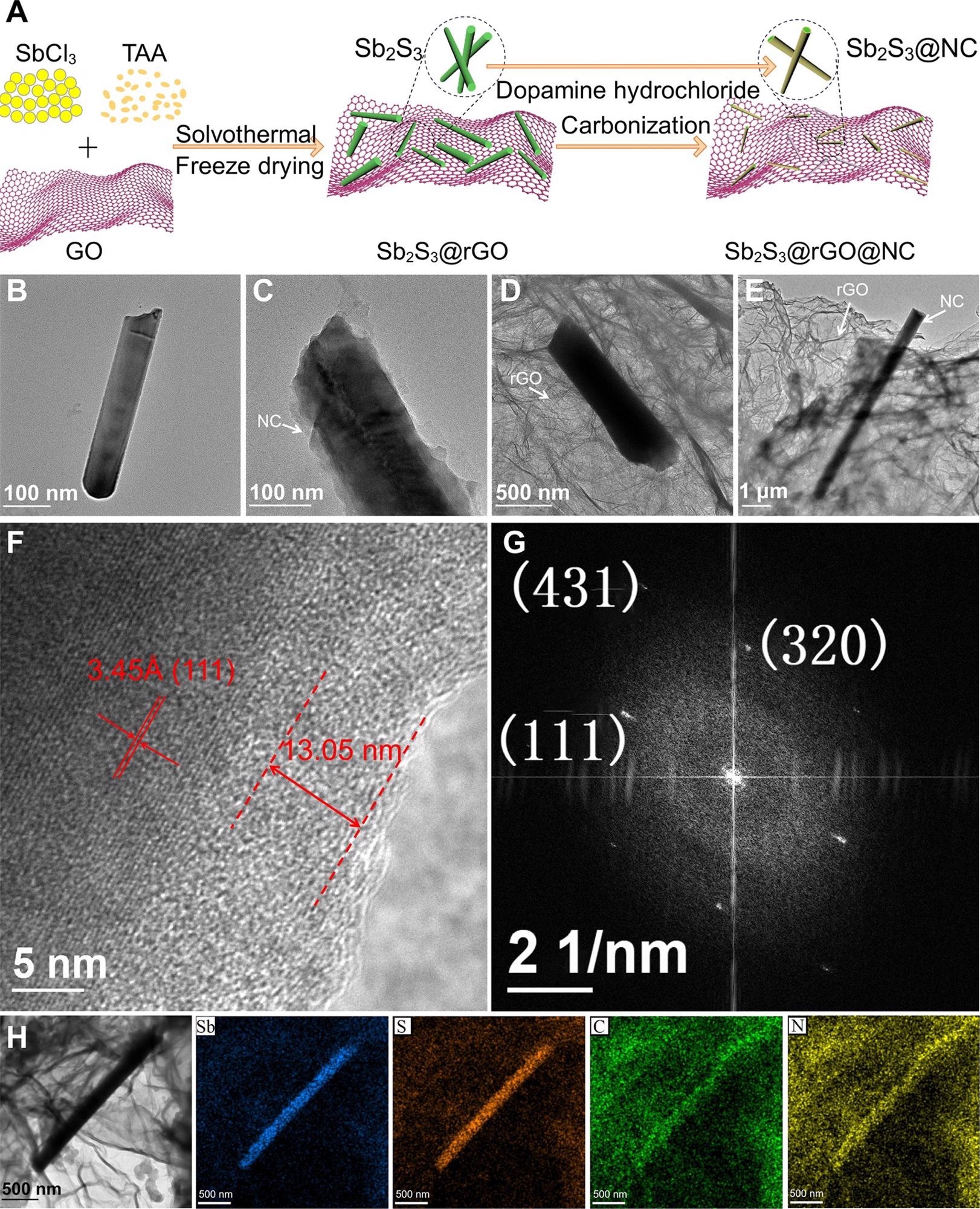
Morphology and microstructure characterization. (A) Schematic of preparation process; TEM images of (B) bare Sb2S3, (C) Sb2S3@NC, (D) Sb2S3@rGO and (E) Sb2S3@rGO@NC; (F) HRTEM, (G) FFT pattern and (H) EDS mapping images of Sb2S3@rGO@NC. Image Credit: Chong, S, ISCIENCE
Additionally, potassium (K) has the second-lowest redox potential of -2.93 V (vs standard hydrogen electrode) after lithium (Li) with a redox potential of -3.03 V. Low redox potential offers high working voltage and high energy storage capacity. Moreover, potassium ion (K+) has the weakest Lewis acidity among alkali metal ions, which imparts K-ion with greater mobility than Li-ion in the electrolyte, thus leading to high power operation.
Also, the Stokes radius of K-ion (3.6 Å) is smaller than that of Li-ion (4.8 Å), which enhances K-ion diffusion kinetics behavior in the electrolyte, and increased the rated capacity of PIBs. PiBs are safer than LiBs due to the lower melting temperature of K than that of Li.
However, despite having the above advantages, the commercial application of PiBs is hindered by the larger ionic radius of K-ion (1.38 Å) than that of Li-ion (0.76 Å) which contributes to large volume expansion during electrochemical cycling.
Suitable Anode Materials for Potassium-Ion Batteries
There are primarily three categories of anodes in electrolyte-based batteries viz. intercalation-type (carbonaceous materials and potassium titanate), conversion reaction (transition metal oxides, sulfides, selenides, phosphides, etc.), and alloy mechanism (bismuth, antimony, tin, etc.).
The carbonaceous materials possess excellent cyclic stability, but low specific capacity. The conversion reaction materials have a high specific capacity, high working voltage, but poor rate capacity and cyclic stability due to large volume expansion and poor conductivity.
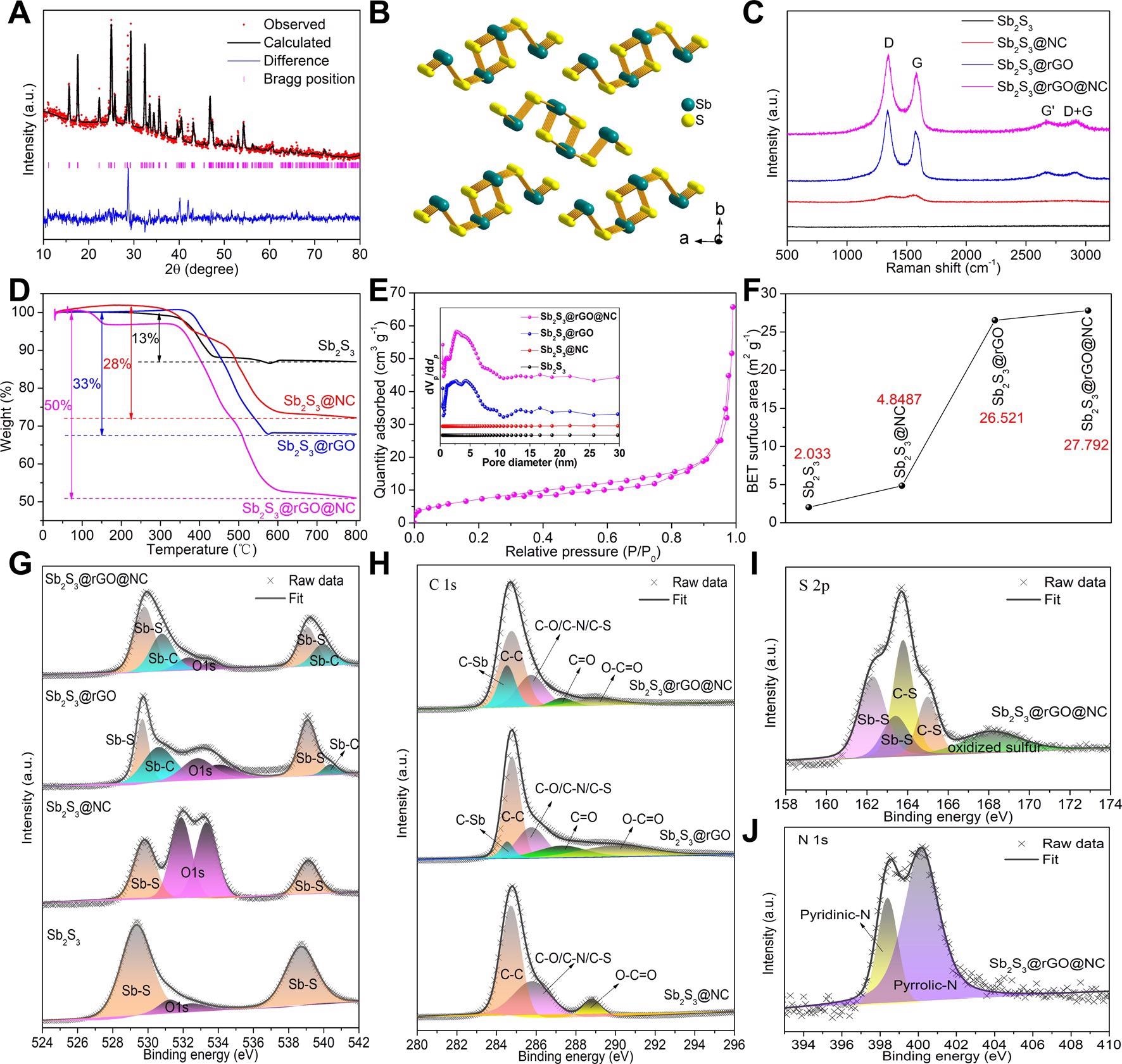
Structure characterization. (A) Refined XRD pattern of Sb2S3@rGO@NC and (B) the corresponding crystal structure model; (C) Raman spectra, (D) TG curves and (E) N2 adsorption-desorption curve of Sb2S3@rGO@NC, the inset of Figure 2E is the pore size distribution of all samples; (F) BET specific surface area of all samples; XPS fitting spectra of (G) Sb 3d, (H) C 1s, (I) S 2p and (J) N 1s. Image Credit: Chong, S, ISCIENCE
Contrary to that, alloying-based materials demonstrate high specific capacity, low working voltage, but high volume expansion, and poor kinetics behavior. Thus, a conversion-alloying-based dual mechanism can be the desired solution to curb these limitations. Sb-based oxides and sulfides (Sb2S3, Sb2O3, Sb2O4, etc.) demonstrate high theoretical capacity, electronic conductivity, reaction reversibility, and mechanical stability than that of pure Sb.
Anode Material Preparation
The researchers wrapped antimony sulfide (Sb2S3) nanorods by reduced graphene oxide (rGO) and nitrogen-doped carbon (NC) through a simple solvothermal process. In this process thioacetamide and antimony chloride were dissolved in the mixed solvent of ethanol and glycerol then graphene oxide was added to the solution and heated for several hours followed by cooling to obtain Sb2S3-rGO.
Further, Sb2S3-rGO and dopamine hydrochloride were added to a solution of trihydroxy methyl aminomethane, hydrogen chloride, and water followed by heating and slow cooling to obtain Sb2S3-rGO-NC.
To fabricate the anode Sb2S3-rGO-NC, PVDF, and acetylene black were mixed and dispersed in N-methyl-2-pyrrolidinone (NMP) followed by coating the solution on copper foils.
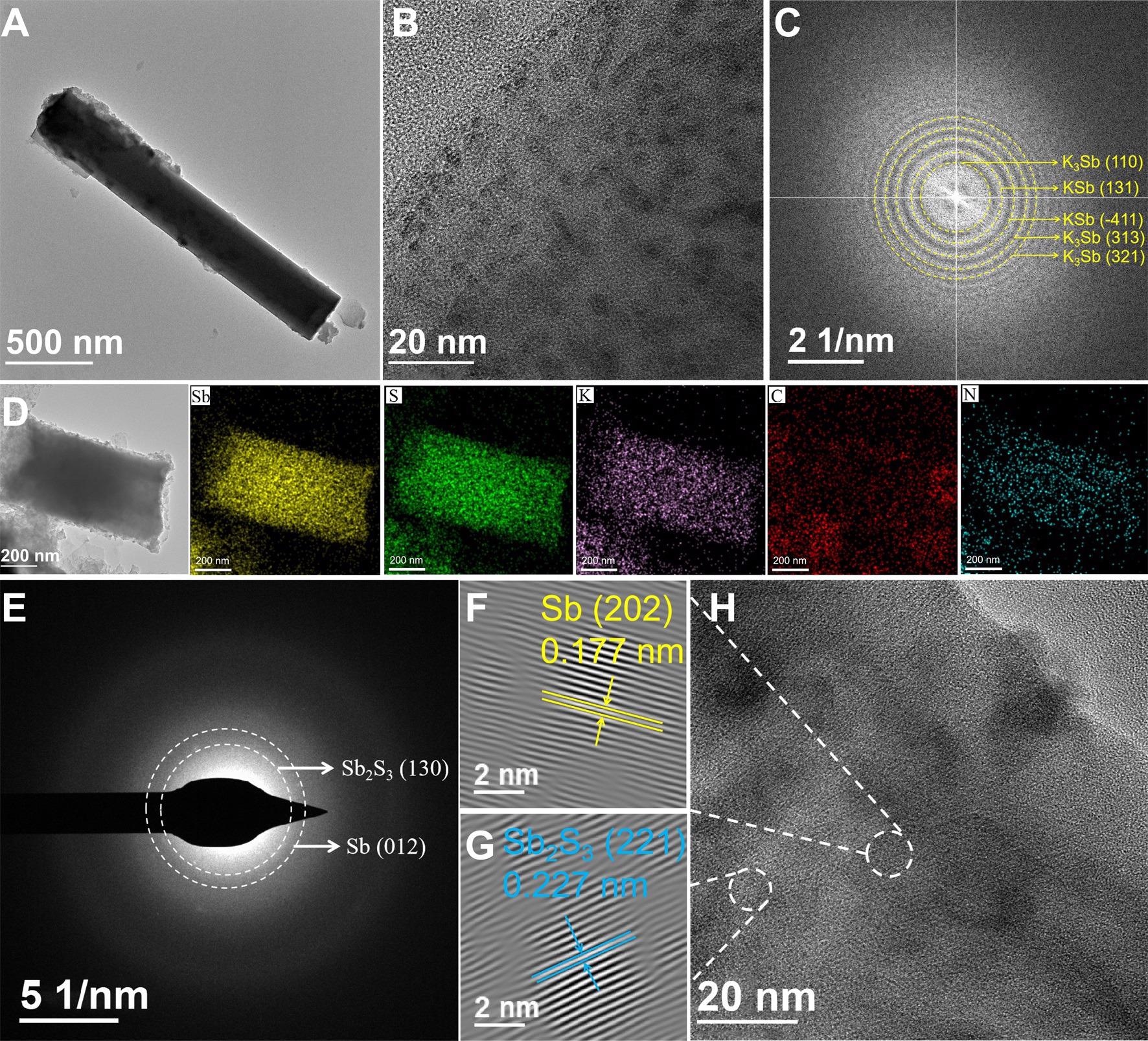
K-ion storage mechanism. (A) TEM image, (B) HRTEM image and (C) FFT pattern of Sb2S3@rGO@NC at the initial fully discharged state; (D) EDS mapping images, (E) SAED pattern, (F-G) inverse FFT patterns and (H) HRTEM image at the initial fully charged state. Image Credit: Chong, S, ISCIENCE
How was the Performance of the Prepared Anode?
The study found that the combination of conversion and alloying reactions was greatly influenced by redox reactions involving Sb as the center and the chemical bonding between Sb and C, thus enhancing the structural stability.
The induced nitrogen enhanced the conductivity of the anode and produced more defects in the carbon layer, thus increasing K-ion adsorption and diffusion between electrodes and electrolytes. The dual nitrogen-doped carbon and a graphene oxide layer formed a buffer space to contain the large volume expansion and effectively enhanced the kinetic process.
Finally, a high reversible capacity of 505.6 mAh·g-1 at 50 mA·g-1, rate capability of 76.7 mAh·g-1 at 1.0 A·g-1, and long cycle life of 200 cycles were achieved for Sb2S3-rGO-NC anode. Therefore, it can be concluded that the prepared conversion-alloying material is a promising anode material for low-cost PiBs.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Chong, S., Qiao, S., Wei, X., Li, T., Yuan, L., Dong, S., Huang, W., Sb2S3- Based Conversion-Alloying Dual Mechanism Anode for Potassium-Ion Batteries, ISCIENCE (2021), 103494, https://www.sciencedirect.com/science/article/pii/S2589004221014656