In a recent paper published in the journal Energies, researchers reviewed the progress in advanced MXene-based nanocomposites, which show excellent performances in electrochemical storage devices such as supercapacitors and metal-ion batteries.
Study: Applications of 2D MXenes for Electrochemical Energy Conversion and Storage. Image Credit: nobeastsofierce/Shutterstock.com
These nanocomposites are a new class of 2D layered transition metal carbides or carbonitrides that possess a large number of functional groups, high electronic conductivity, and excellent hydrophilicity and dispersibility in aqueous solutions.
What are MXenes?
The development of new classes of advanced two-dimensional (2D) layered materials, including graphene, phosphorene, and MoS2 has promoted tremendous technological progress in energy storage systems due to their extraordinary properties, namely, the large interlayer spaces within the layered structures that facilitate intercalation and diffusion of ions, suppress volume change during charge/discharge process, high carrier mobility, and electrical conductivities.
![(a) The elements of the periodic table for MAX phases. (b) Structures of M2AX, M3AX2, and M4AX3 phases [41].](https://d12oja0ew7x0i8.cloudfront.net/images/news/ImageForNews_57627_16390457957155446.jpg)
(a) The elements of the periodic table for MAX phases. (b) Structures of M2AX, M3AX2, and M4AX3 phases. Image Credit: Ji, C et al., Energies
2D MXenes viz. Ti2CTx, Ti3CNTx, and V2CTx have adjustable composition, hydrophilic behavior, conductivity, thermal conductivity, tunable bandgap, and excellent mechanical strength, which make them preferable for high and flexible energy density storage systems.
However, they easily agglomerate into multilayer structures owing to strong Van der Waals forces between the layers, which lead to the decrease in the interlayer space and slow redox or intercalation/deintercalation reactions. Hence, many studies are going on to enlarge the space between the interlayers of MXenes.
Substantial efforts have also been made in methodologies of patterned MXene-based composite film or MXene-based conductive ink for fabricating more types of energy storage devices, which includes vacuum filtration, mask-assisted filtration, screen printing, extrusion printing technique, and directly writing.
Synthesis of MXenes
The MXene flakes are fabricated by using hydrofluoric acid (HF) or a mixed solution of lithium fluoride (LiF) and hydrochloric acid (HCl) to selectively extract the “A” element from the ternary “MAX” phase, where the M represents transition metals, the A represents IIIA or IVA group elements, and the X represents carbon and/or nitrogen.
The MXenes possess an accordion-like hexagonal lattice structure, which forms due to the original metallic backbone, weak M-A bond, and strong M-X bond. Additionally, during the bond breaking and binding processes, some surface terminal groups are formed, which gives suitable interlayer spacing and active sites.
Hydrofluoric acid (HF) etching is the most effective process to selectively react with the “A” layer atoms and continual out-diffusion to exfoliate the MAX phase. Nevertheless, the HF reagent is a highly corrosive and hazardous poison, and hence, many other mild etchants such as NH4HF2 or a mixture of LiF and HCl have been developed.
The properties of the final MXenes (e.g., metallic, semi-metallic) and semiconducting types directly depend upon the preparation process and the functional groups present. The conversion of MAX phase to MXene sheets is achieved through three approaches, namely, wet-chemical etching in hydrofluoric acid, in situ HF-forming method, and freeze-and-thaw-assisted (FAT) method.
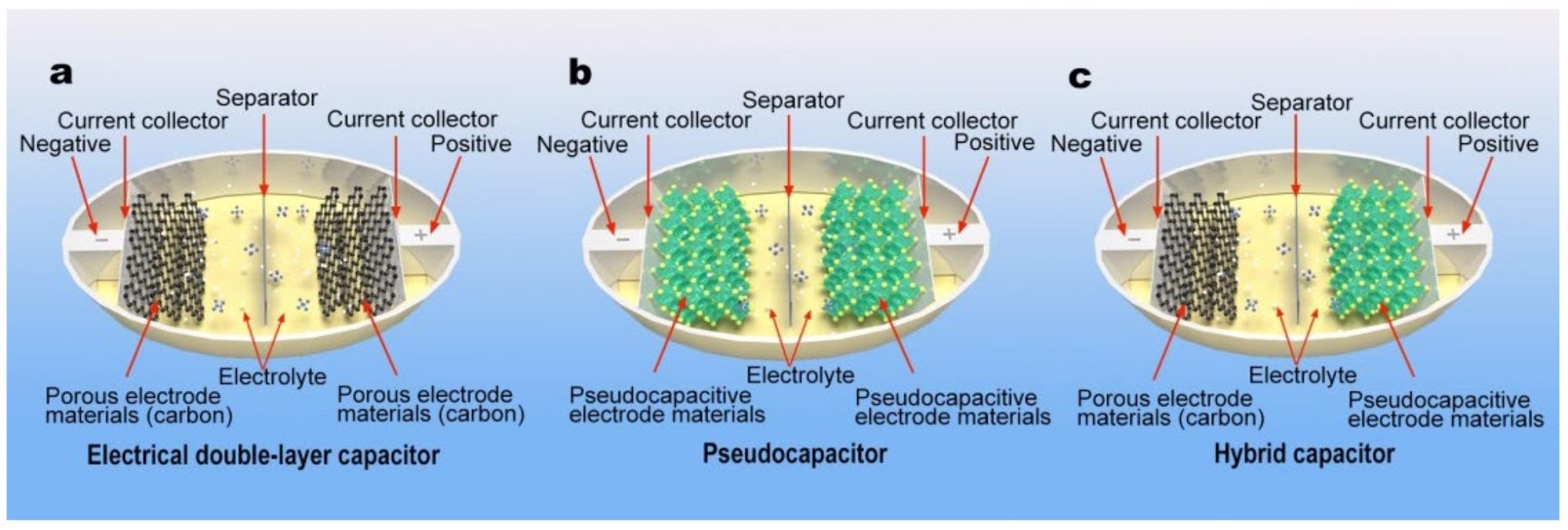
Schematic illustrations of (a) a EDLC with porous carbon materials for each electrode, (b) a pseudocapacitor with transition metal-related material for each electrode, and (c) asymmetric hybrid capacitors utilizing a porous carbon negative electrode and a transition metal oxide positive electrode. Image Credit: Ji, C et al., Energies
Applications of MXenes
MXenes display great potential in lithium-ion batteries (LIB) due to their large available surface areas for electrolyte ion adsorption, satisfactory electrical conductivity, desirable ion transfer within the inter-layers, and rapid surface redox reaction.
Moreover, MXenes such as Ti3C2 are used as a functional host matrix for integrating other materials such as germanium oxide, iron oxide, and tin sulfate for further enhancing the overall energy storage performance of LIB. The deposited SnO2 on the MXene can effectively suppress the degradation of MXene while the thin inactive layer of HfO2 would serve as an artificial solid-electrolyte interphase (SEI) layer for enhancing the cycling stability.
Moreover, MXenes with 3D heterostructure exhibit high capacitance with excellent cyclic stability, which makes them a promising supercapacitor (SC) material.
A Ti3C2/CuS composite as the positive electrode in alkaline electrolyte with a potential of 0–0.6 V delivers a capacity of 169.5 C/g at 1 A/g with a capacity retention of 90.5% at 5 A/g after 5000 long-term cycles. The SC fabricated by the Ti3C2/CuS positive electrode and Ti3C2 negative electrode at a voltage range of 0–1.1 V exhibits a specific capacitance of 49.3 F/g to 0–1.5 V with a maximum energy density of 15.4 Wh/kg and capacitance retention of 82.4% after 5000 cycles.
![Schematic illustration of the synthesis process for the MXene/MPFs film via interlayer hydrogen bond [75].](https://d12oja0ew7x0i8.cloudfront.net/images/news/ImageForNews_57627_16390458279515183.jpg)
Schematic illustration of the synthesis process for the MXene/MPFs film via interlayer hydrogen bond. Image Credit: Ji, C et al., Energies
Conclusions
To conclude, MXene-based composite films or fibers with geometric flexibility and multifunctionality made them preferable as electrodes for SCs and anode materials for zinc- and lithium-ion batteries. Moreover, the electrochemical performance of MXene-based materials is enhanced by enlarging the interlayer of MXene, modifying the chemical structure, and constructing the nanostructures. Hence, MXenes have a promising future in flexible, wearable, and lightweight energy storage devices.
Reference
Ji, C., Cui, H., Mi, H., Yang, S., Applications of 2D MXenes for Electrochemical Energy Conversion and Storage. Energies 2021, 14, 8183. https://www.mdpi.com/1996-1073/14/23/8183/htm
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.