Latest design approaches and operational burner optimization for diverse renewable fuels are required. One option is to combine or switch the burning of natural fuels such as natural gas with fuels derived from other sources (biogas plants, and pyrolysis plants). Alternative fuels having a lower heating value (LHV) are often burned in burners that are particularly suited for that fuel's combustion. The presence of inert gases reduces the LHV in this type of fuel.
To maintain appropriate energy outputs in furnaces or burners, a greater volume of fuel must be delivered into the combustor (as opposed to normal gas consumption). Conventional stoves that were originally built for the burning of natural gasoline and hydrocarbons with comparable LHV are not typically meant to be utilized in conjunction with low-calorific fuels. As a result, changing the combustion layout is frequently required.
Diluted Gaseous Fuels
Several research teams have looked at the ignition of diluted hydrocarbons, including petroleum as the primary flammable element. When water and water vapor are introduced to the flame, they greatly reduce NOx emissions in the flue gas. Furthermore, with the injection of steam, a shift in the breakdown of methane into OH radicals was detected. Furthermore, the addition of steam to the flame resulted in a drop in the OH percentage in the flame; hence, at the same time, a decrease in CH radicals was seen, resulting in the suppression of quick NOx. It was also discovered that the impact of dilution by CO2 is greater than that of dilution by water, implying that when regenerated flue gas is employed, CO2 predominates.
Experiment Setup
In the recent research, three distinct burner models were employed and their emission, flame properties, flame intensities, and heat flow were all evaluated. The goal of this study was to uncover the capabilities of fuel interdependency in traditional process furnaces and to identify limits while raising the number of inert chemicals in the fuel. The burner test facility was the tool for conducting combustion experiments. The temperature capacity of the furnaces was set to 500 kW for the burning experiment using low-calorific fuels.

Semi-industrial burner testing facility. Image Credit: Hudák et al., Energies
The experiment began with the reference fuel—in this example, natural gas. All of the low-calorific fuel findings were compared to this reference fuel. Low-calorific fuels are distinguished by the presence of non-combustible components that reduce the LHV, yet there is at least one flammable ingredient. The mixing station ensured that inert gases and flammable substances were properly mixed.

Model of a mixing station. Image Credit: Hudák et al., Energies
Research Findings
Studies with Burner A indicated that the burner's structure is insufficient for a concentration of more than 10 mN3/h. The burner head, which was initially built for noble fuels, was limited in its ability to disperse additional fuel. It seemed obvious that a larger flow rate would be attainable with more fuel overpressure. Burner B's shape was created with a greater thermal production in mind than Burner A's. As a result, more inert gases may be added to the fuel. This burner burns all of the fuel in the main phase and uses the supplementary distribution of air to reduce NOx emissions.
Burner C was intended for enhanced flow rates at the burner's head, thus the fire was volatile during natural gas burning without any N2 or CO2. As a result, fuel blending with air mixture was inadequate, and a lengthy flame was observed.
When inert gas was supplied, recorded NOx in the exhaust gases began to fall, correlating to a drop in temperature. The shift was also minimal since the concentration of inert gases introduced was rather low. When inert gas is contained in the fuel, it quickly affects the temperature, which reduces in general. With the addition of inert gas, the temperature rises. This might be due to the greater operating speed of the fuel, which leads to more mixing.
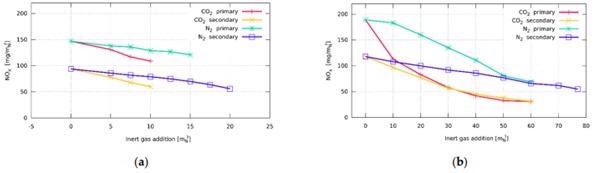
Influence of inert gas addition on total NOx emissions: (a) burner with staged fuel distribution—Burner A; (b) burner with staged air distribution—Burner B. Image Credit: Hudák et al., Energies
When both N2 and CO2 are added, it is possible to identify an enhanced flame at the center (higher temperatures), but with a tiny decline towards the assembly's end. The injection of the inert gas caused a temperature drop throughout the whole flame, indicating that CO2 absorbed more heat than N2. The inclusion of inert gas resulted in just a little modification in the heat flux distribution in the chamber. Furthermore, there were essentially no significant changes in the overall heat flow.
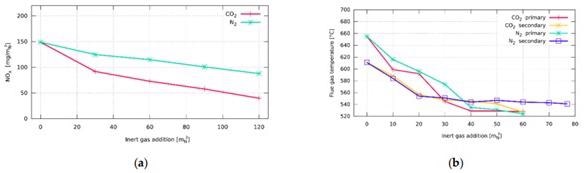
Influence of inert gas addition on flue gas temperature: (a) burner with staged fuel distribution—Burner A; (b) burner with staged air distribution—Burner B. Image Credit: Hudák et al., Energies
To summarize, adding inert gas to conventional energy sources can be helpful; it can boost the effectiveness of flow blending, which affects directly thermal performance, but determining the tipping point is difficult. It is more advantageous to connect the burners with two separate sets of nozzles when co-combusting fuels such as natural gas and biogas. If a little loss in thermal power is not a concern, a single set of nozzles might be employed.
Further Reading
Hudák et al. The Effect of Inert Fuel Compounds on Flame Characteristics. Energies. 2022; 15(1). 262. Available at: https://www.mdpi.com/1996-1073/15/1/262
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.