In a recent study published in the journal Materials, researchers from Poland conducted thermogravimetric analysis and scanning calorimetry of plastics used in the food industry. The results indicated that the highest thermal effect occurred in polyethylene terephthalate decomposition out of all the analyzed plastics samples.

Study: Thermal Analysis of Plastics Used in the Food Industry. Image Credit: Nataliia Maksymenko/Shutterstock.com
Plastic Packaging Waste and Air Pollution
Plastic production has surpassed 300 million tonnes due to its superior performance and wide range of applications. Around 40% of all plastics are used in packaging, with 41% devoted to food and beverage packaging. Plastic is frequently used for packaging food due to its resistance to physical and chemical effects, lightweight, and inexpensive manufacturing costs. Frequently used packaging plastics are polyethylene terephthalate, polyethylene, polypropylene, and polystyrene plastics.
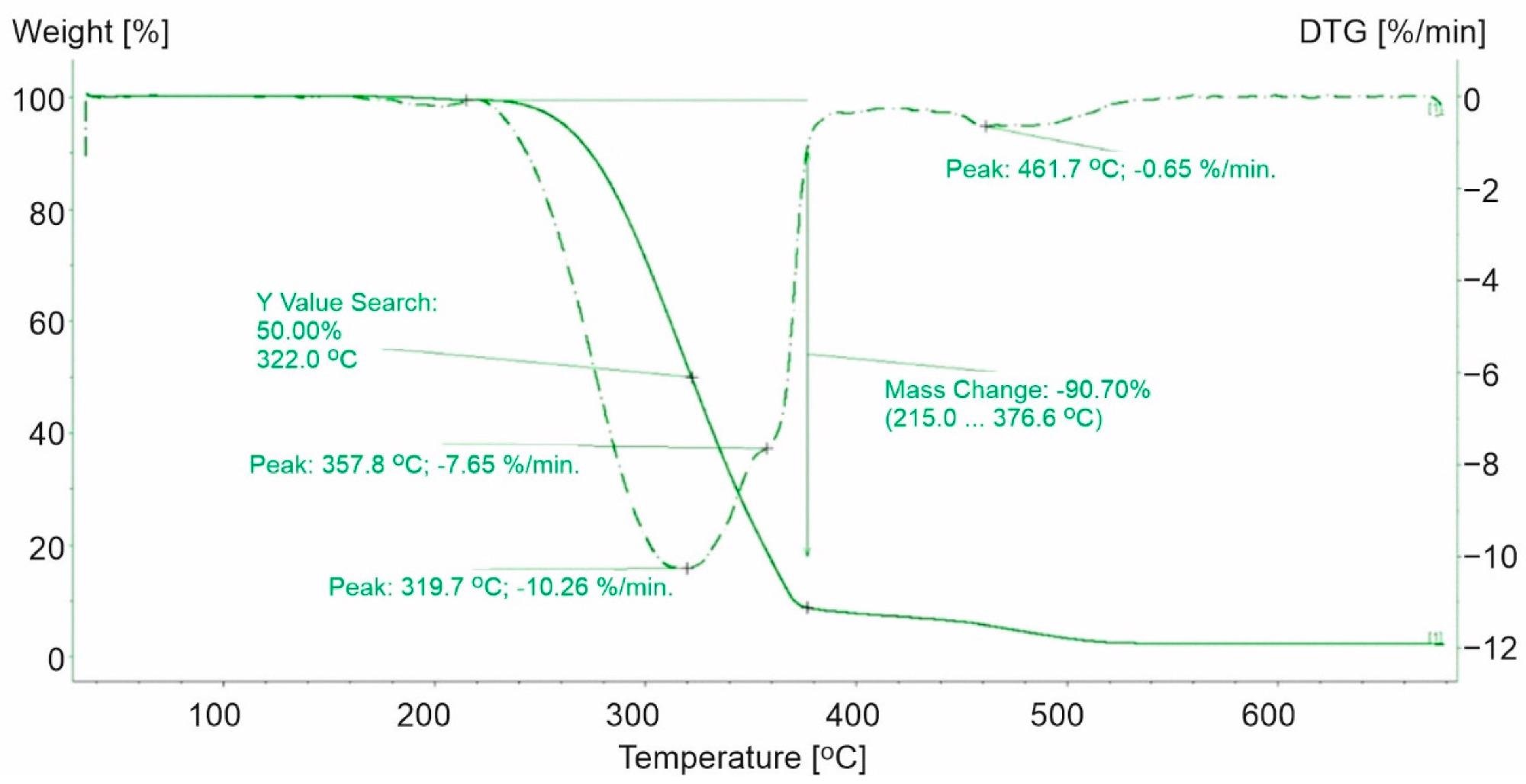
TG and DTG curves for PP-1. The green line presents TG curve, while the green dot line presents DTG curve. Image Credit: Majder-Łopatka, M et al., Materials
However, the burning of plastic debris in landfills intensifies the fire and is the primary cause of air pollution. Most of the time, municipal solid trash comprising approximately 12% plastic is burned, releasing harmful substances into the atmosphere such as polycyclic aromatic hydrocarbons, dioxins, furans, and polychlorinated biphenyls.
Additionally, a detailed understanding of thermal analysis is critical for assessing fire risks and defining polymer processing and recycling conditions. Thermal analysis has evolved into the dominant method for determining the combustion properties of various materials.
In the present study, the researchers investigated the thermal degradation method of plastic packaging waste that is typically deposited in landfills. A series of thermogravimetric analyses and differential scanning calorimetry tests (TG-DSC) were conducted under precise temperature circumstances on polypropylene, polystyrene, and polyethylene terephthalate plastics to determine the thermal degradation path of the plastics.
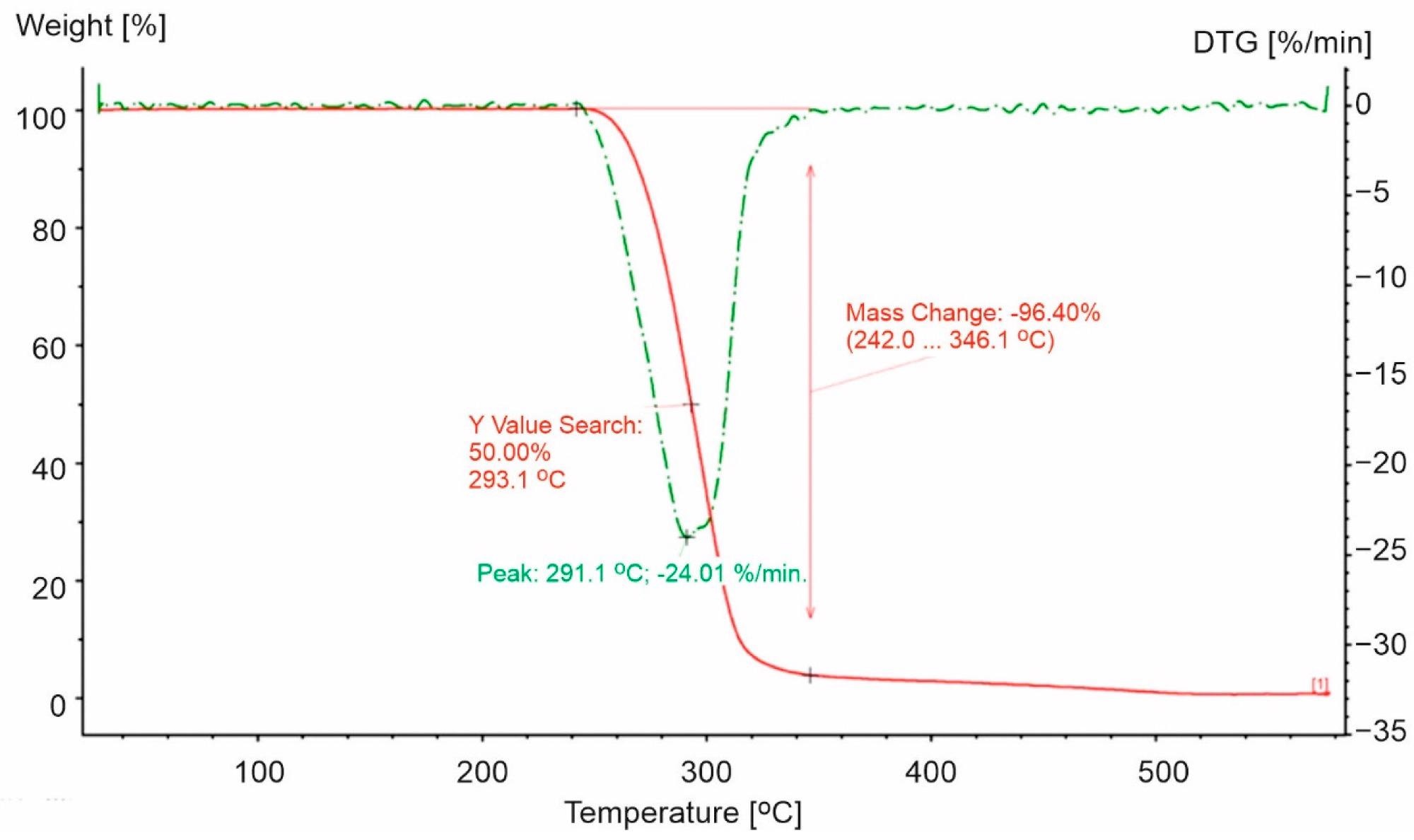
TG and DTG curves for PP-0 in an oxidizing atmosphere (20% O2). The red line presents TG curve, while the green dot line presents DTG curve. Image Credit: Majder-Łopatka, M et al., Materials
Methodology
Researchers used five types of plastic packaging materials used in the food industry. For the study, three samples of polypropylene (PP), one sample of polystyrene (PS), and one sample of polyethylene terephthalate (PET). In addition to this, the sample of pure polypropylene (PP-0) was also examined.
The total weight of the examined samples was 10 mg. The samples were examined in open alumina crucibles on the TG-DSC sample carrier, and they were cut out of plastic packets with no imprints. They were then placed in the crucible to cover the entire bottom area of the pan.
Results
The thermal stability results indicated that among the plastics tested, the PP plastics had the lowest thermal stability and PET thermoplastics had the highest thermal stability. With regard to the thermal stability of PP and PS, the relative weakness of C-H bonds on the tertiary carbon atom may explain the pace of degradation of PP and PS. The dissolution of C-H bonds on the tertiary carbon atom bond allows the stability of hydroperoxide, which converts into stable tertiary alcohol, and increases the ease of separating the hydrogen atom from the benzylic carbon atom in PS. As a result, PS will degrade at higher temperatures than PP.
The researchers discovered the lowest amount of heat generated during the thermo-oxidation of PS-based plastics. This is caused by the creation of a styrene molecule during heating, which is caused by the high stability of aromatic ring bonds and volatility escaping from the combustion zone without oxidation. The proposed mechanism of thermo-oxidation indicated that a phenyl radical is formed during break down, and its decomposition is considerably easier than that of the neutral aromatic ring.
Furthermore, the results reveal that the PP samples can consist of PP and PE copolymers. Compared to the base polymer, polystyrene, and PET-based plastics, the PP-based plastics demonstrated a maximum rate of weight loss.
In comparison to the base polymer and polystyrene and PET-based plastics, the PP-based plastics demonstrated a reduced maximum rate of weight loss and a shift in the 50% weight loss temperature toward lower temperature values.
The study also revealed that PS-based packaging decomposes at a higher temperature than pure polystyrene. In PET packaging, the opposite trend was observed. With regard to PET, faster degradation was seen for PET, which could be attributed to the existence of more extended aliphatic parts in the molecule containing a large number of methylene units, increasing the probability of breaking the C-C bond and ethylene fragment in PET.
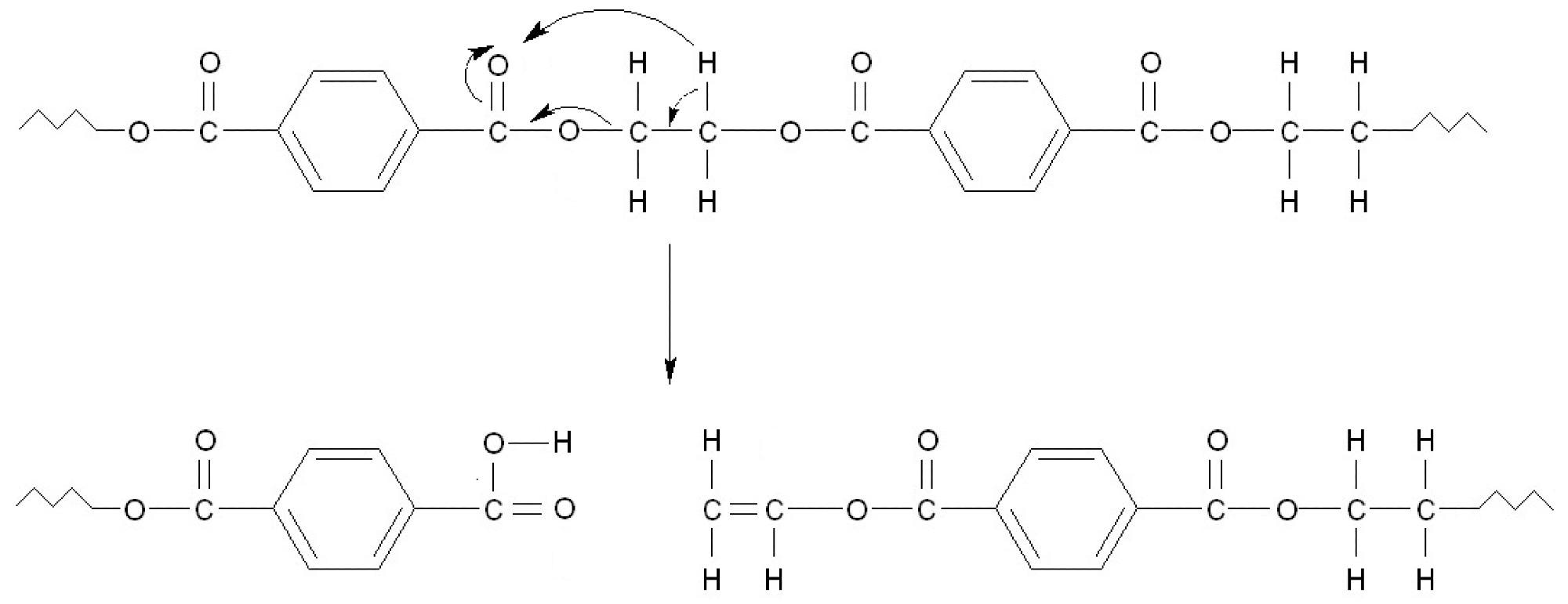
Schematic PET decomposition according to Norrish type reaction II. As a result, during subsequent reactions, terephthalic acid and derivatives containing vinyl group are formed. Image Credit: Majder-Łopatka, M et al., Materials
Conclusions
Plastic packaging continues to account for a sizable amount of waste collected in municipal landfills and poses a substantial risk in the case of a fire. The thermal stability of these materials is mainly determined by the polymer type, the additives utilized, and their structure. This research demonstrated that the most significant heat effect occurs during PET decomposition, which results in the formation of phenyl radical, in which the C-H bonds break rapidly.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Majder-Łopatka, M.; Wesierski, T.; Ankowski, A.; Ratajczak, K.; Duralski, D.; Piechota-Polanczyk, A.; Polanczyk, A. Thermal Analysis of Plastics Used in the Food Industry. Materials 2022, 15, 248. https://www.mdpi.com/1996-1944/15/1/248