The latest study published in the journal Metals looked at the electrochemical impact of tensile-stressed and non-stressed carbon steels, as well as the impact of tension on steel passivation and rusting behavior in a modeled concrete pore fluid.

Study: Galvanic Corrosion Study between Tensile-Stressed and Non-Stressed Carbon Steels in Simulated Concrete Pore Solution. Image Credit: P. Thungsarn
Carbon Steel and Its Utilization
Carbon steel is an iron-carbon alloy with up to 2.1 weight percent carbon. There is no minimal defined proportion of additional alloying in carbon steels, however the frequently included element is manganese. Carbon steel is divided into three types based on its calorific value: low-carbon steel, medium-carbon steel, and high-carbon steel. Low carbon steels are frequently utilized in vehicle body parts, structural forms, pipelines, buildings, and food canisters.
Medium-carbon steels are commonly used for rail tracks, locomotive tires, engine components, gearboxes, and engineering products due to their high toughness, wear resistance, and durability. High-carbon steels are utilized in metal cutting, springs, great-strength wire, and castings due to their high friction coefficient and stiffness.
Effect of Stress
External stress increases forceful chemical infiltration owing to the production of fractures at aggregate/paste contacts (i.e., continual microcracks), reducing the performance of the concrete. Despite the damage in concrete, stress affects the corrosive rate of steel. Under stress, the erosion dynamics can change, potentially because of localized lattice distortion and increased kinetic energy.
When steel is exposed to the alkaline conditions of cement, a latent coating develops on its surface. Tensile stress may cause microscopic cracks in the steel's passive oxide coating, whereas compressive stress may cause delamination between the protective film and the steel substrates.
As a result, delamination to the oxide layer of steel under stress is possible. In terms of the effect of compressive stress, steel under stress conditions outperforms unloaded steel when subjected to a chloride-laden atmosphere.
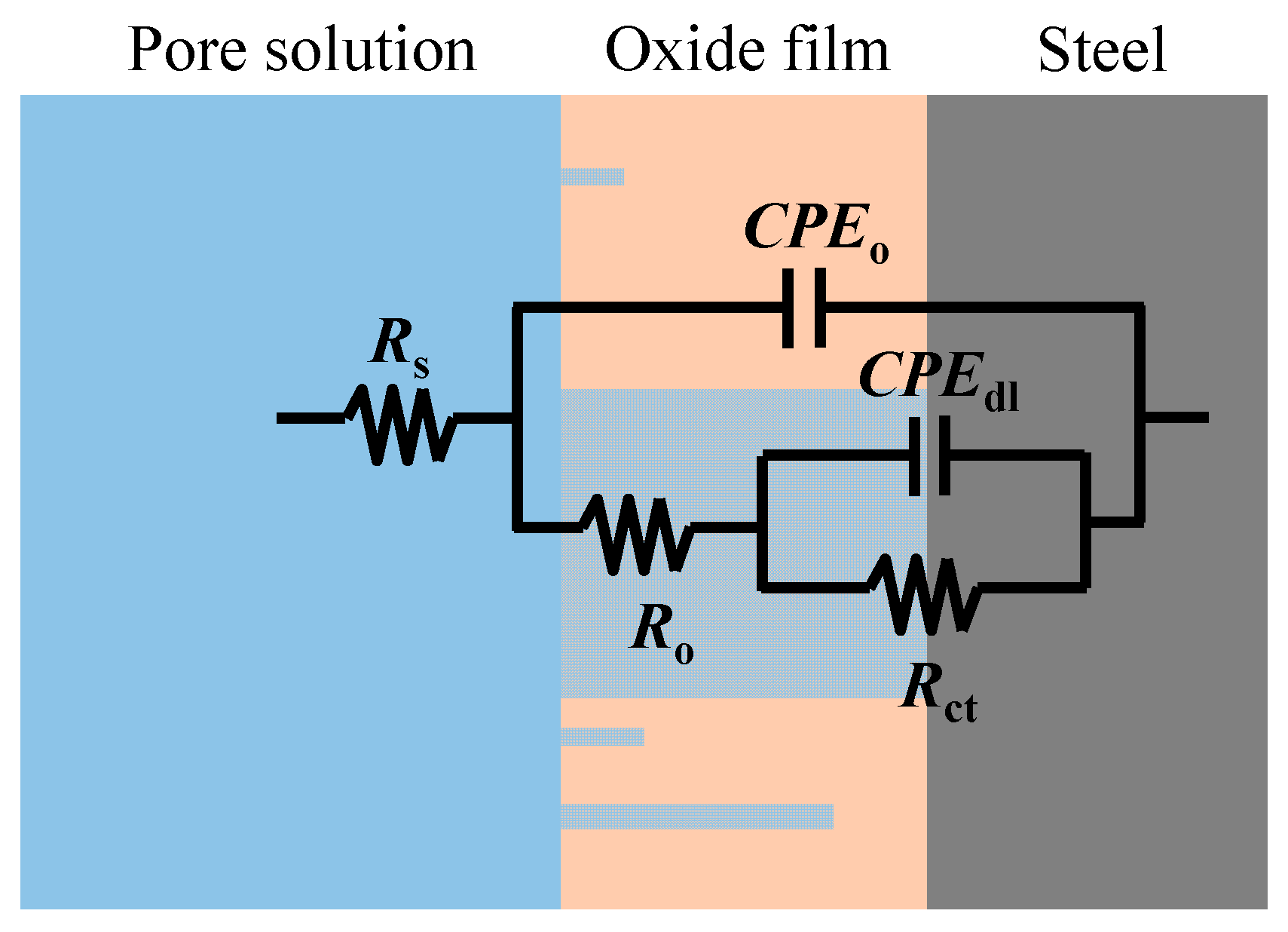
The equivalent circuit used to simulate the behavior of steel in the concrete pore solution. Image Credit: Dong et. al., Metals
Galvanic Effect
The galvanic effect refers to corrosive harm caused by the coupling of two different metals in the presence of an electrolyte. It happens when two (or more) different metals make electrical contact beneath the water. When two different metals are submerged in a conductive fluid and electronically linked, galvanic corrosion develops.
One metal is shielded (the cathode), while the other (the anode) is eroded. To minimize the galvanic effect, using materials with equal corrosive capabilities and disconnecting the electrical connection using insulation is effective. Apart from this, the inclusion of corrosion inhibitors, coating the materials, and the inclusion of sacrificial material may play their part in minimizing this harmful phenomenon.
Experimental Conditions
C-shape samples have been frequently employed in assessing metallic stress corrosion. The exposed portion in a C-shape carbon steel alloys experienced the highest tensile stress value.
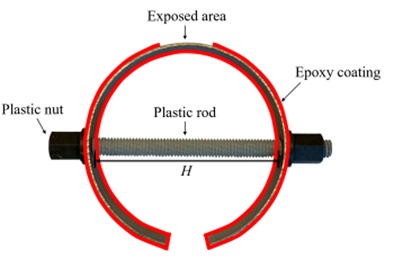
Schematic illustration of the C-shape carbon steel. Image Credit: Dong et. al., Metals
The faces of the samples were covered with three coats of epoxy resin, except the exposed region. Unique samples were used to create sets with stress levels of (σ y) of 0%, 33%, 55%, and 100% of yield stress (y), labeled as A0, A3, A5, and A10, respectively. To imitate the concrete microenvironment, a synthetic concrete pore solution (SCPS) was employed as a solution. Specimens' open circuit potential (OCP) values were monitored every three days using a saturated calomel electrode (SCE) as the test set. The oxidation and corrosion properties of both specific samples were investigated using the electrochemical impedance spectroscopy (EIS) method.
Research Findings
During the SCPS passivation of steel, Fe was first oxidized to produce complexes of Fe(II) and Fe(III) oxides. The findings of the time-dependent variations in the resistivity of the oxide film (Ro) and the impedance of the charge transfer (R ct) indicated that the inclusion of chlorides reduced both Ro and Rct. After the injection of chlorides, the charge transport impedance of tensile-stressed steels dropped precipitously, finally yielding a similar or even lower value of R ct than that of non-stressed steel (A 0).
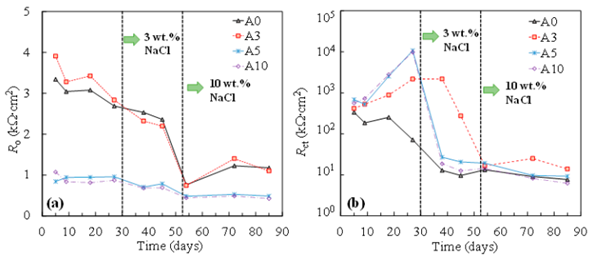
Resistance of oxide film (a) and charge transfer (b) of steel specimen at various stress levels. Image Credit: Dong et. al., Metals
The decrease of passive film resistance (Ro) in the concrete matrix was noticed as the tensile stress increased. In both the chloride-free and chloride-contaminated SCPSs, Ro declined as the tensile value of stress rose. After adding 3% wt. percent sodium chlorides to individual steel specimens, a significant decrease in charge transfer resistance (Rct) was frequently found.
Through electrochemistry, the latest study explored the galvanic corrosion of tensile-stressed and non-stressed steels in an SCPS, as well as the impact of tensile stress on the corrosion behaviors of steel. In the future, a long-term examination of galvanic corrosion between strained and non-stressed steels is required.
Further Reading
Dong et. al. Galvanic Corrosion Study between Tensile-Stressed and Non-Stressed Carbon Steels in Simulated Concrete Pore Solution. Metals. 2022; 12(1). 98. Available at: https://www.mdpi.com/2075-4701/12/1/98
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.