The science of catalysis — the expedition of a chemical reaction — is probably not the best-known branch of study, but it is fitted into the fabric of modern society.
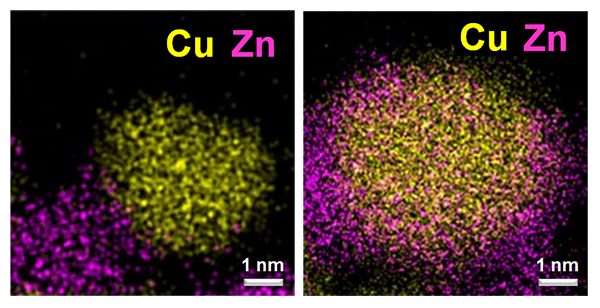 Scanning transmission electron microscopy images of catalysts metallic copper (yellow) and zinc oxide (pink/orange). In the image on the left, metallic Cu and Zn oxide are mostly present as separate particles after activation with H2. The image on the right shows Zn oxide decorating metallic Cu particles after “induced activation” with H2/CH3OH/H2O. Image Credit: Images courtesy of Xuan Tang and Prof. Sheng Dai, East China University of Science and Technology.
Scanning transmission electron microscopy images of catalysts metallic copper (yellow) and zinc oxide (pink/orange). In the image on the left, metallic Cu and Zn oxide are mostly present as separate particles after activation with H2. The image on the right shows Zn oxide decorating metallic Cu particles after “induced activation” with H2/CH3OH/H2O. Image Credit: Images courtesy of Xuan Tang and Prof. Sheng Dai, East China University of Science and Technology.
The development and production of chemicals, pharmaceuticals, fuels and other goods are based on catalysis. Catalysis plays a crucial role in energy generation and the reduction of humanity’s effect on the surrounding.
It is included in the manufacturing of around 25% of all industrial products in the United States. From a consumer’s point of view, if a thing is created, worn, lived in, played with, driven upon or otherwise utilized by people, catalysis probably plays a basic role in its origin story.
The study performed in the field of catalysis allows new and enhanced products and highly efficient approaches of doing and manufacturing, well, almost all that you can imagine.
However, with these deep entanglements in the world around us, progress in industrial catalysis can be expensive in a macroeconomic sense — wholesale variations that need a “rip and replace” strategy do not sit well with firms and provide chains that power and provision the modern economy.
Scientists from Lehigh University, in collaboration with a partnership from the East China University of Science and Technology (ECUST), suggest a novel technique of considerably improving the catalytic efficiency of materials earlier in broad commercial usage, a process they have called “induced activation.” The study was published on January 20th, 2022, in the journal Nature Catalysis.
The research team assisted by the National Natural Science Foundation of China and the U.S. Department of Energy’s Office of Science, contains Israel E. Wachs, the G. Whitney Snyder Professor of Chemical and Biomolecular Engineering at Lehigh University.
The team also included Ph.D. student Tiancheng Pu of Lehigh’s Operando Molecular Spectroscopy and Catalysis Research Lab, and Minghui Zhu, a 2016 Lehigh Ph.D. who currently serves as a professor of chemical engineering at ECUST.
Other collaborating ECUST scientists include Didi Li, Fang Xu, Xuan Tang, Sheng Dai, Xianglin Liu, Pengfei Tian, Fuzhen Xuan and Zhi Xu.
Induced Activation: A Game Changer in the Control of Catalytic Surface
The surface structure of heterogeneous catalysts is closely associated with their catalytic performance. Current efforts for structural modification mainly focus on improving catalyst synthesis.
Israel E. Wachs, G. Whitney Snyder Professor, Chemical and Biomolecular Engineering, Lehigh University
Wachs added, “Induced activation, on the other hand, takes a different approach — manipulating the catalyst surface by controlling the composition of reducing agents at the catalyst activation stage where the catalyst is transformed to its optimum state.”
The researchers say that the usage of the “tried and true” industrial catalytic material copper or zinc oxide/aluminum oxide (Cu/ZnO/AlO3) allows firms to take advantage of the discovery without the need for expensive retooling.
This development effectively doubles the catalytic efficiency of these materials, enhancing their productivity and extending the life of the catalyst. And importantly, induced activation can provide significant benefit to industry without shutting down a chemical plant—or the building of a new and costly one.
Israel E. Wachs, G. Whitney Snyder Professor, Chemical and Biomolecular Engineering, Lehigh University
Journal Reference:
Li, D., et al. (2022) Induced activation of the commercial Cu/ZnO/ Al2O3 catalyst for the steam reforming of methanol. Nature Catalysis. doi.org/10.1038/s41929-021-00729-4.