In a recent study published in the journal Materials, researchers from China synthesized nitrogen (N)-doped bamboo-based activated carbon (NBAC) for carbon dioxide (CO2) capture and sequestration using a facile one-step mix-and-heat method.

Study: In Situ Dry Chemical Synthesis of Nitrogen-Doped Activated Carbon from Bamboo Charcoal for Carbon Dioxide Adsorption. Image Credit: somchaiP/Shutterstock.com
The two ingredients for the activation mixture were bamboo charcoal (BC) and sodamide (NaNH2) (SA). The CO2 adsorption capacity of NBAC was 3.68–4.95 mmol/g and 2.49–3.52 mmol/g at 0 ℃ and 25 ℃, respectively.
Moreover, the low initial isosteric heat of adsorption, higher selectivity (15-17) of CO2 over nitrogen (N2), and efficient renewability of the synthesized NBAC make it a promising material for CO2 capturing devices.
Activated Carbon for CO2 Capturing
CO2 adsorbing materials viz. activated carbon (AC), metal oxides, molecular sieve, and metal-organic frameworks (MOFs) are energy-efficient solutions for CO2 capture, utilization, and sequestration.
Among these, activated carbon has the advantages of high special pore structure, high specific surface area, chemical stability, renew-ability, simple synthesis, and high adsorption capacity. Another benefit of AC is that it can be synthesized from multiple organic bioresources by chemical activation.
Activation through potassium hydroxide (KOH), SA, and N-doping are known for the synthesis of functionalized porous carbon materials for enhanced CO2 adsorption.
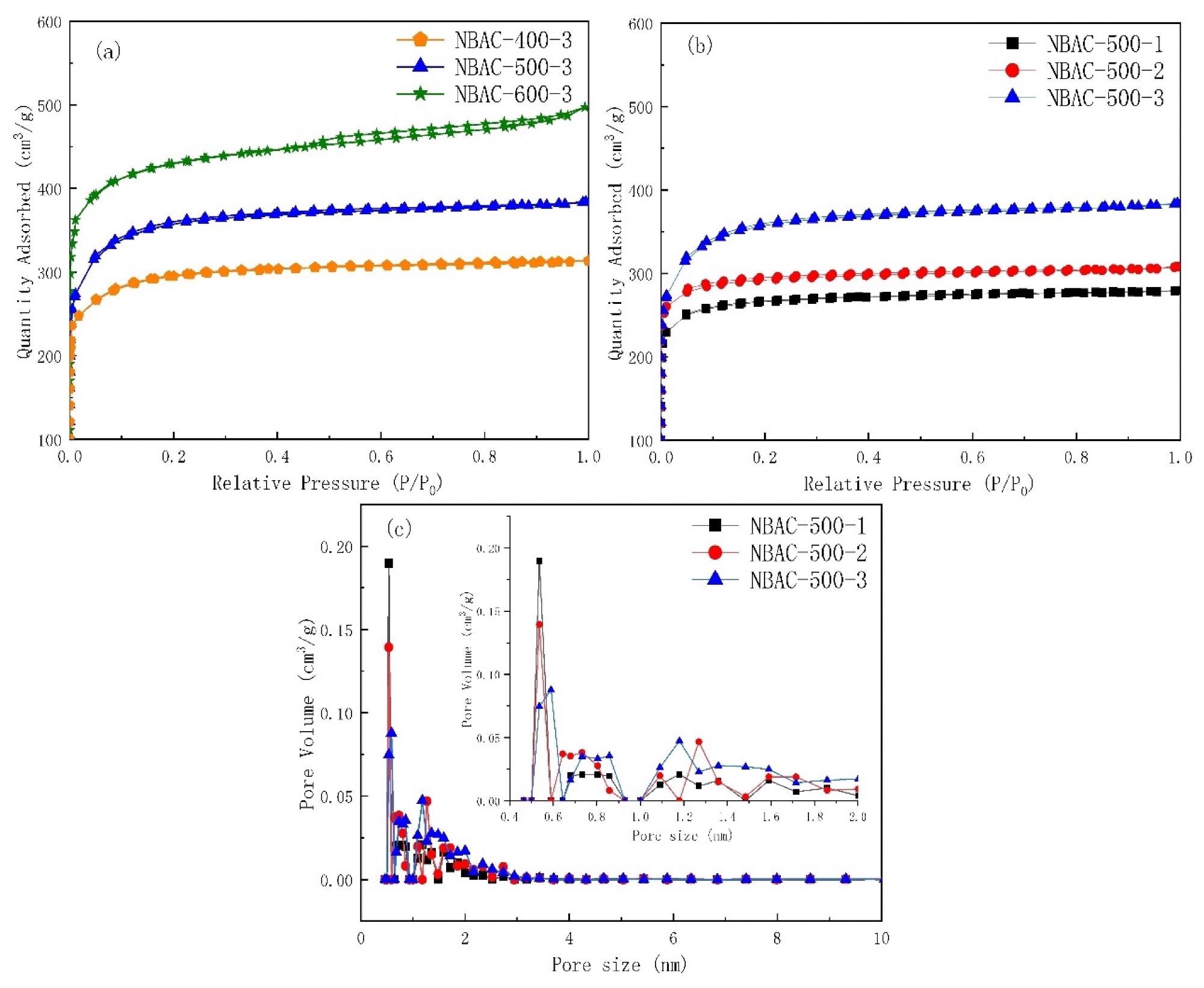
N2 adsorption–desorption isotherm (a,b) of N-doped bamboo-based activated carbon synthesized by sodamide activation and its pore size distribution (c). Image Credit: Ying, W et al., Materials
BC is the final solid residue of the pyrolysis of bamboo in the absence of oxygen. Bamboo is an abundantly cultivated plant in tropical and temperate regions of China. Other than carbon, bamboo also contains a small amount of nitrogen, silicon, and other minerals which helps in the synthesis of more functionalized activated carbon.
About the Study
In this study, researchers synthesized NBAC using a facile one-step mix-and-heat method in which BC was mixed with SA at three blending ratios of 1:1, 2:1, and 3:1 followed by heating in a tubular furnace under N2 atmosphere and in a medium temperature range of 400-600 ℃, which was much lower than that of other conventional chemical activation processes that require temperature up to 800 ℃ and beyond.
The prepared NBAC powders were cooled down to the ambient temperature and rinsed using 10% diluted hydrochloric acid (HCl) to neutralize and remove the residue.
Subsequently, all NBAC samples were characterized, and their CO2 adsorption isotherms were measured at a pressure of 1.0 bar and temperatures of 0 ℃ and 25 ℃. To compare the gas adsorption selectivity between CO2 and N2, the N2 adsorption isotherm was measured at the pressure of 1.0 bar and 25 ℃.
The isosteric heat of adsorption was calculated using the Clausius–Clapeyron equation, whereas the adsorption heat and adsorption selectivity were calculated using the single-site Langmuir–Freundlich equation with ideal adsorbed solution theory (IAST).
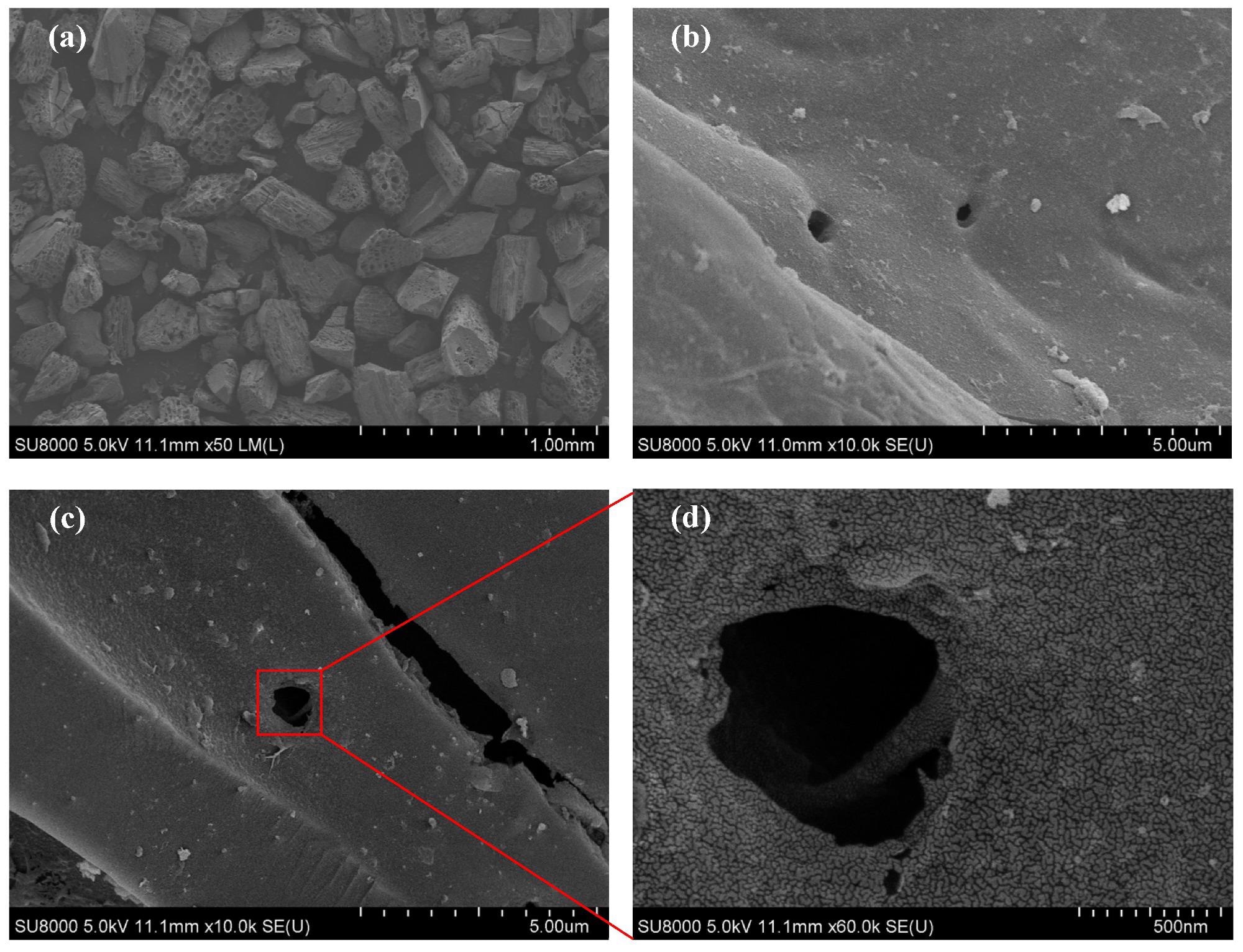
SEM patterns of bamboo charcoal (a,b) and N-doped bamboo-based activated carbon (c,d). Image Credit: Ying, W et al., Materials
Observations
Scanning electron microscopy (SEM) images revealed that bamboo AC (BAC) had massive trenches and holes compared to the relatively smooth surface of BC owing to the effectiveness of chemical etching by SA. Additionally, the elemental analysis showed that the N2 content of untreated BC was 0.26%, whereas it was 2.35% for the NBAC sample that was activated at 600 ℃ with a BC/SA composition ratio of 3:1. This ten-time increase in N2 content signified successful N-doping of samples.
The X-ray photoelectron spectroscopy (XPS) graph showed that at 600 ℃ the partial pyrrolic N (N-5) and pyridinic N (N-6) phases transformed into a more thermodynamically stable quaternary N (N-Q) phase, which was undesirable. Hence, N-doping activation at 500 ℃ was optimal to induce functional groups that promoted CO2 capture.
Moreover, the maximum CO2 adsorption capacity of NBAC was 3.68–4.95 mmol/g and 2.49–3.52 mmol/g at 0 ℃ and 25 ℃, respectively. All NBAC samples activated at 500 ℃ with different BC/SA compositions exhibited adsorption selectivity between 15.03 to 16.97 for CO2. The samples with a lower concentration of SA had more selectivity.
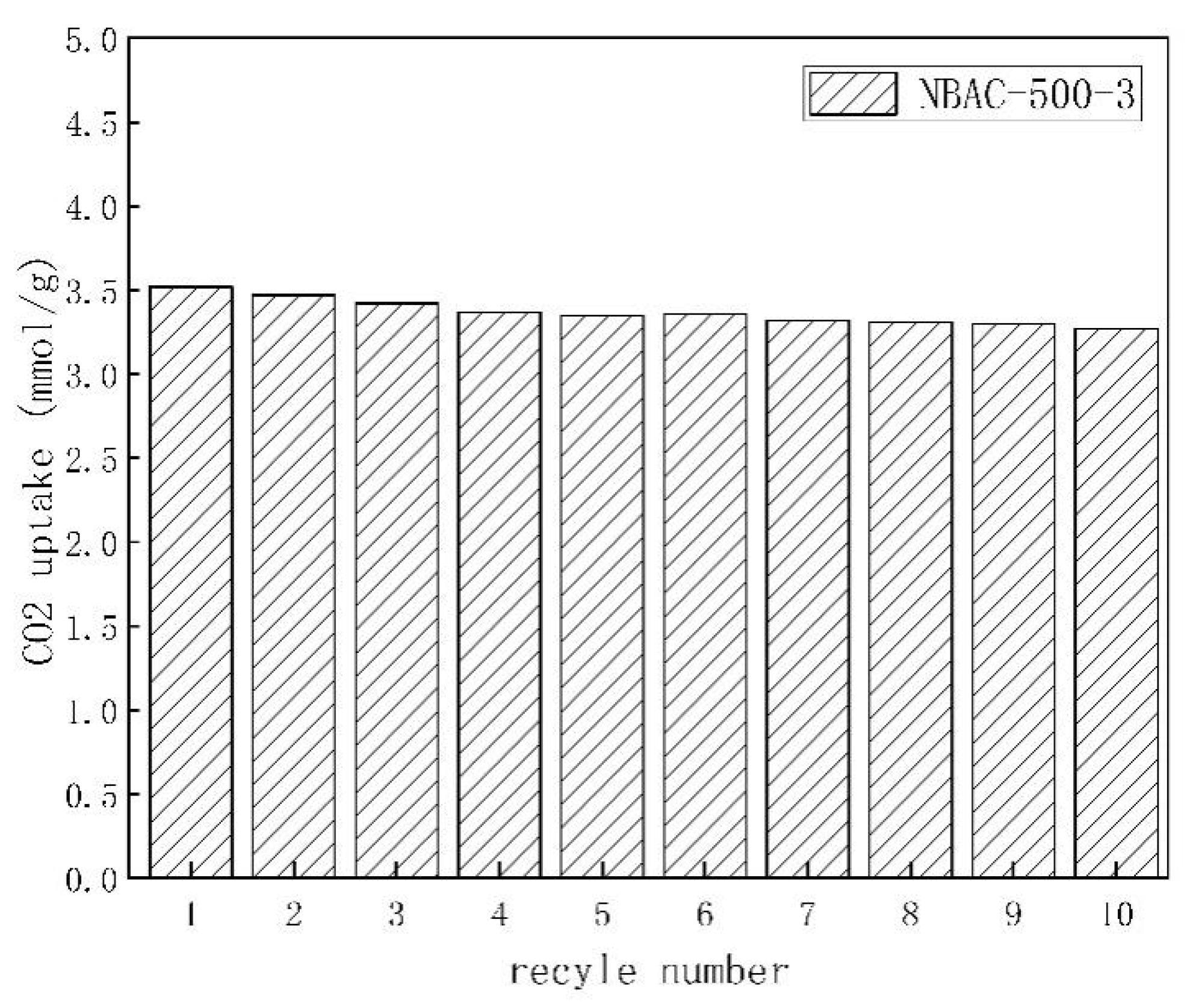
Adsorption/desorption experimental results for NBAC synthesized at 500 °C. Image Credit: Ying, W et al., Materials
Conclusions
To conclude, in this study researchers synthesized NBAC using a facile one-step mix-and-heat method. They used BC obtained from the pyrolysis of natural bamboo as the source of unactivated carbon and activated it in a tubular furnace at a lower temperature range than other conventional chemical activation processes.
The N-doping and activation occurred simultaneously inside the furnace using SA as the activator. The resulting NBAC had 10 times more N2 content than unprocessed BC. Other than that, high CO2 adsorption selectivity and renewability make NBAC a promising low-cost CO2 capturing material.
Reference
Ying, W., Tian, S., Liu, H., Zhou, Z., Kapeso, G., Zhong, J., Zhang, W., In Situ Dry Chemical Synthesis of Nitrogen-Doped Activated Carbon from Bamboo Charcoal for Carbon Dioxide Adsorption. Materials, 2022, 15, 763. https://www.mdpi.com/1996-1944/15/3/763
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.