.jpg) By Susha Cheriyedath, M.Sc.Reviewed by Skyla BailyJan 24 2022
By Susha Cheriyedath, M.Sc.Reviewed by Skyla BailyJan 24 2022A team of researchers recently published a paper in the journal Materials that reviewed ecological methods to synthesize nicotinic acid from commercial raw materials with a special focus on methods that could be used in potential industrial applications.

Study: Methods to Produce Nicotinic Acid with Potential Industrial Applications. Image Credit: Gorodenkoff/Shutterstock.com
Significance of Nicotinic Acid
Nicotinic acid is a pyridine carboxylic acid that can be naturally and typically used as an antipelagic agent. Annually, a substantial share of nicotinic acid produced across the world is used as a feed additive and food additive, while the rest is used in industrial and pharmaceutical applications.
Nicotinic acid is an essential nutrient for animals and humans. In humans, nicotinic acid deficiency can cause a large number of disorders such as dementia, diarrhea, and dermatitis, while in animals, deficiency of nicotinic acid can impair their growth and reproduction.
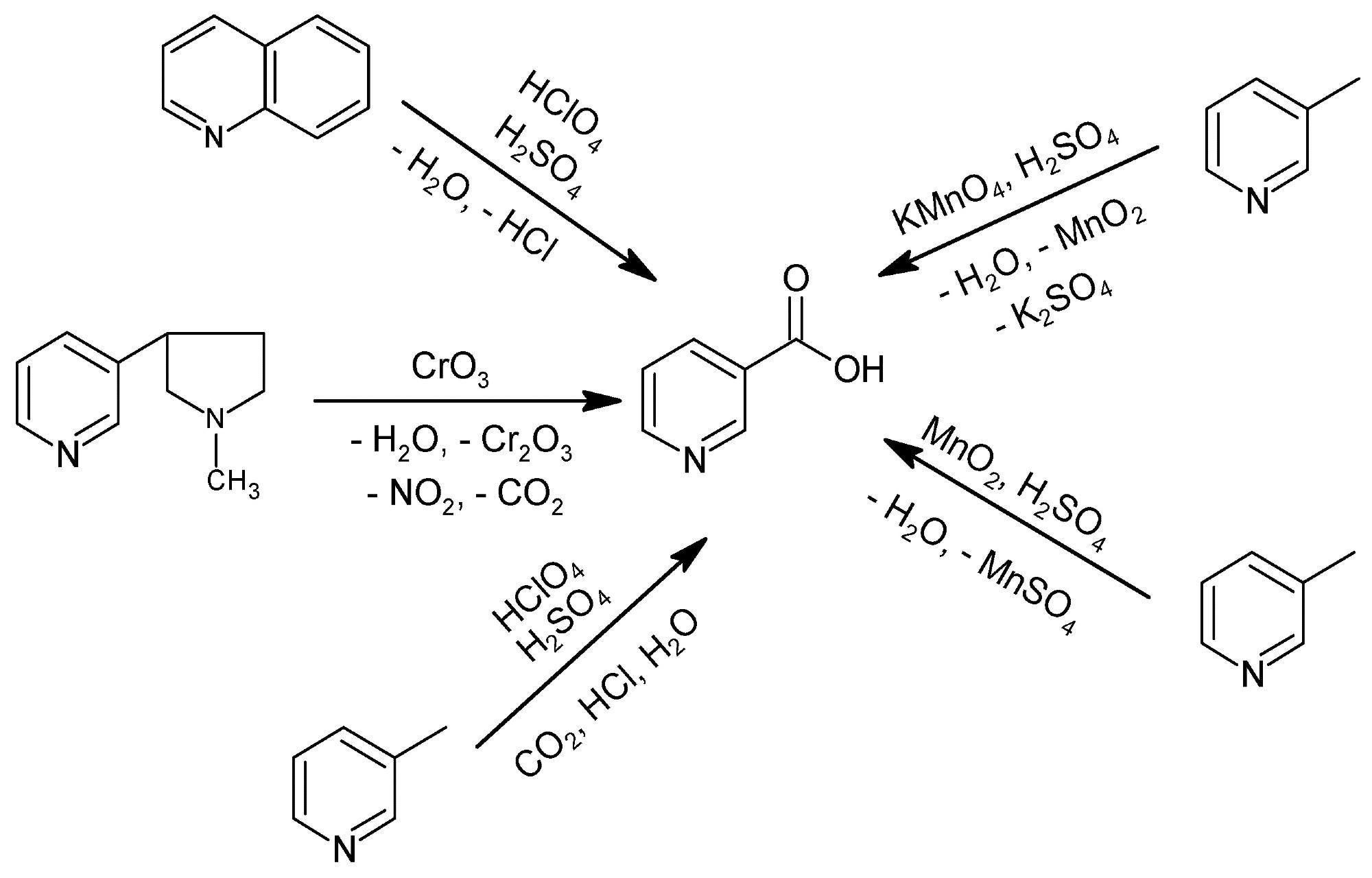
Historical methods for NA production. Image Credit: Lisicki, D et al., Materials
Hence, nicotinic acid is extensively used as feed additives for farm animals, domestic animals, and fish. For humans, nicotinic acid is available in the form of dietary supplements and mixtures and an extended-release prescription drug.
In the industry, nicotinic acid is used for tin plating in a sulfate bath, chemical polishing of steel under high temperatures, and recovering silver from slag melting. Additionally, nicotinic acid is also used for preparing magnetically modified iron oxide (F3O4), or heterogenized silica-based catalysts, and as a chelating agent in the production of VIII and VIB metal catalysts for hydrocracking.
Background
Although nicotinic acid can be produced from tryptophan by animals and plants, the acid is not completely bioavailable. Almost 90% of nicotinic acid is produced synthetically from 5-ethyl-2-methylpyridine or 3-methylpyridine. Industrial production of nicotinic acid involves the oxidation of 5-ethyl-2-methylpyridine with nitric acid (HNO3). However, the process generates nitrous oxide (N2O), a toxic greenhouse gas, as one of the by-products.
Thus, identifying a new technology for the production of nicotinic acid on an industrial scale is necessary that can meet the requirements of green chemistry and does not act as a burden on the environment. In this paper, researchers performed a literature review on ecological methods of producing nicotinic acid from commercially available raw materials such as 5-ethyl-2-methylpyridine and 3-methylpyridine.
Production Methods
Historically, nicotinic acid was produced by the oxidation of quinoline or 3-methylpyridine with stoichiometric oxidizing agents such as perchloric acid (HClO4), potassium permanganate (KMnO4), or manganese dioxide (MnO2) in the presence of sulfuric acid (H2SO4). However, the production method was highly inefficient due to high wastage, low atom economy, unit operations, and multiple processes. The use of HClO4 as an oxidizing agent leads to a considerable wastage and the generation of hydrochloric acid (HCl), a dangerous oxidizing agent, as a by-product.
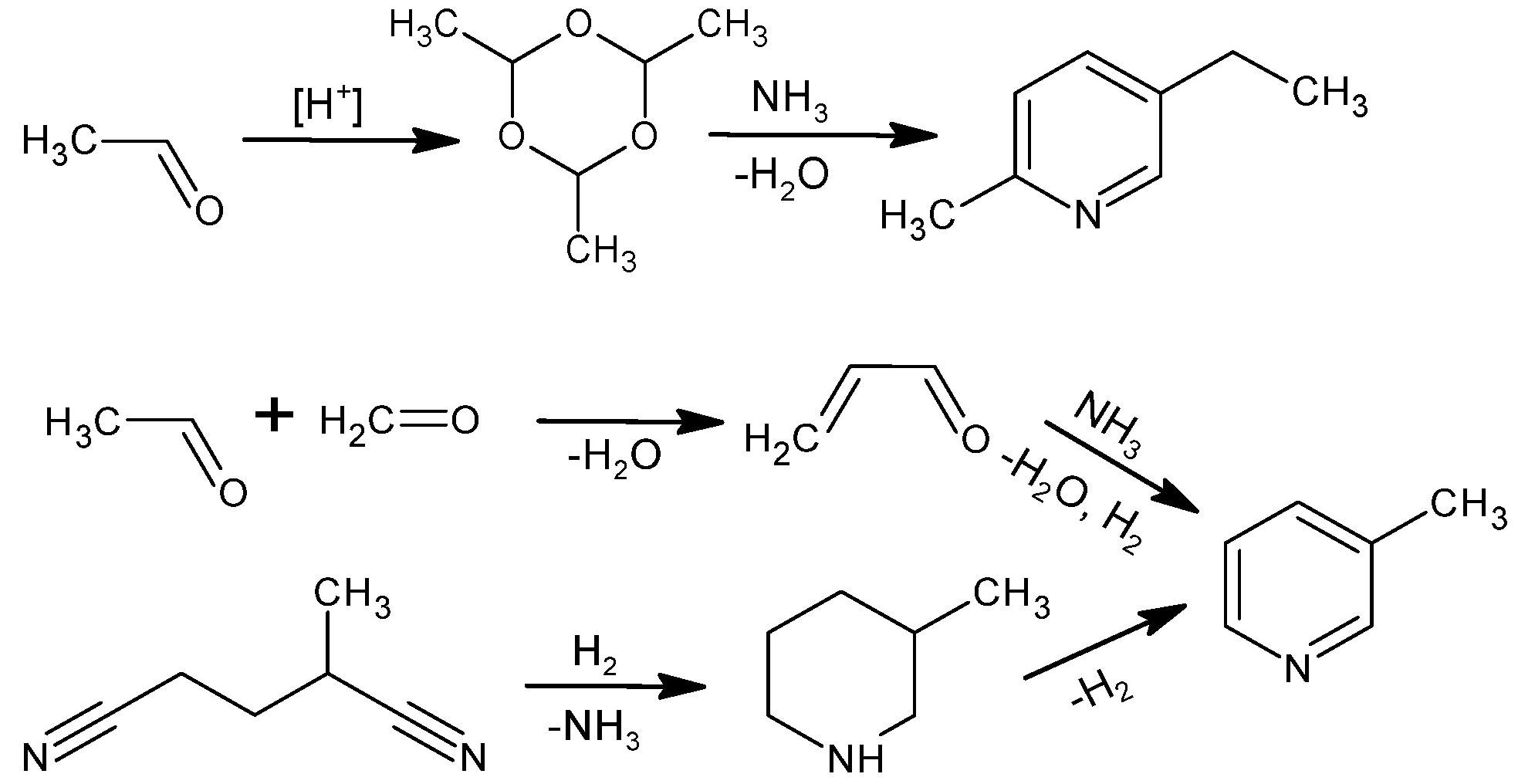
Industrial methods to produce 5-ethyl-2-methylpyridine or 3-methylpyridine. Image Credit: Lisicki, D et al., Materials
The industrial production of nicotinic acid is primarily based on the oxidation reaction of 3-methylpyridine or 5-ethyl-2-methylpyridine with HNO3. Major benefits of this production method include a substantially high conversion rate and production efficiency, and easy availability of raw materials. However, the excessive use of HNO3 as an oxidant leads to the generation of N2O, one of the major greenhouse gases. Additionally, the method requires high pressure and temperature, longer oxidation time, and has a low atom economy.
Nicotinic acid is also produced by the oxidation of 3-methylpyridine in the liquid phase with eco-friendly oxidizing agents such as organic hydroperoxides and oxygen using bromine (Br) or carbon monoxide (CO) as catalysts.
Prominent benefits of this production method include substantially high conversion rate and production efficiency, high atom economy, harmless by-products, low-temperature requirement compared to the industrial method, low wastage, and the use of oxygen as an oxidant, However, the method requires a longer oxidation time, a high pressure, the use of a polar solvent, and a highly corrosive reaction environment.
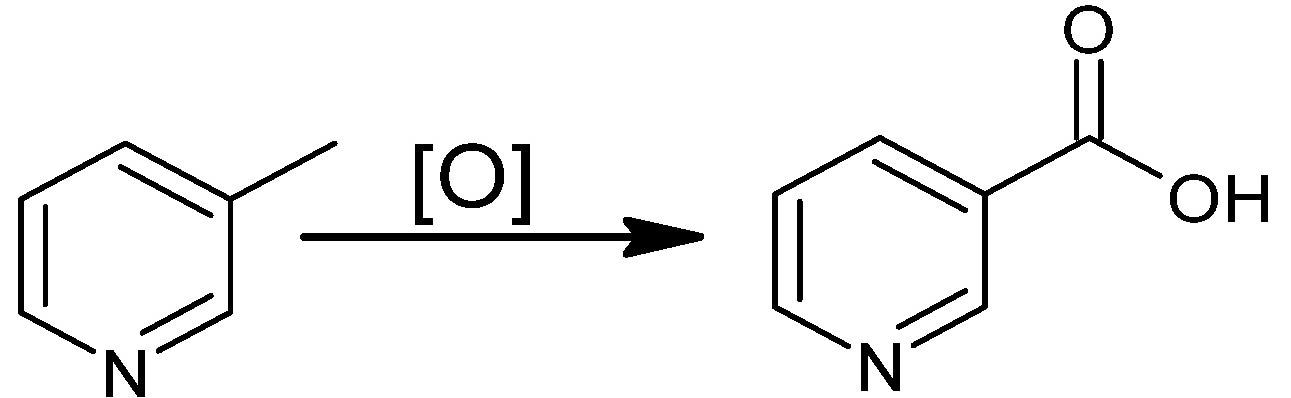
Oxidation of 3-picoline in the liquid phase. Image Credit: Lisicki, D et al., Materials
In another method, the oxidation of 3-methylpyridine in the gas phase with air using heterogeneous catalysts produces nicotinic acid. This method has several benefits that include high conversion rate and efficiency, harmless by-products, oxidation time less than 10 minutes, need for extremely low pressure, and a high atom economy. However, the method requires a slightly corrosive reaction environment and a significantly high temperature. Additionally, nicotinic acid produced by this method often contains impurities.
The oxidative ammonolysis of 3-methylpyridine in the gas phase to 3-cyanopyridine followed by hydrolysis to nicotinic acid amide is another industrial-scale method for producing nicotinic acid. However, this method has low efficiency, low atomic yield, and low atom economy. Additionally, this production method is more complex than the other methods.
Significance
This literature review evaluates relevant aspects of all nicotinic acid production methods. Based on this review, the nicotinic acid production method involving the oxidation of 3-methylpyridine in the liquid phase satisfies most of the requirements of green chemistry and is implementable on an industrial scale. This method provides a high conversion rate and yield, is comparatively simple, and requires a few unit operations than other methods. However, proper corrosion inhibitors are required for this method due to the high corrosivity of the Br or Co catalytic system.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Lisicki, D., Nowak, K., Orli´nska, B. Methods to Produce Nicotinic Acid with Potential Industrial Applications. Materials 2022, 15, 765. https://www.mdpi.com/1996-1944/15/3/765