On a theoretical basis, iron that rusts in water should not be subjected to corrosion when it comes in contact with an “inert” supercritical fluid of carbon dioxide. However, it does.
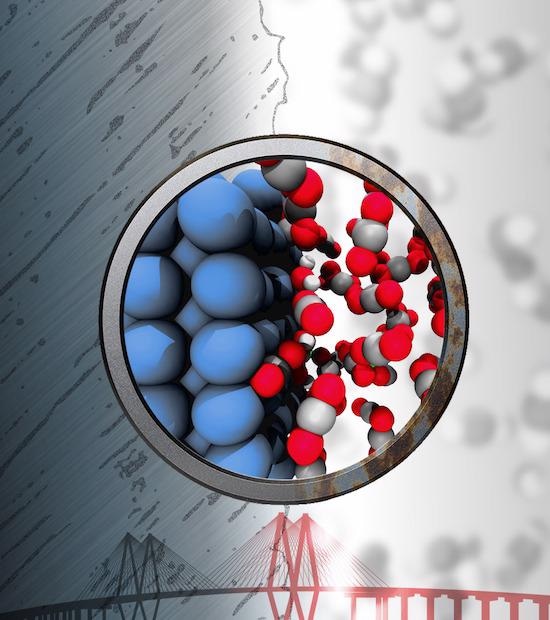 Iron (blue) can react with trace amounts of water to produce corrosive chemicals despite being bathed in “inert” supercritical fluids of carbon dioxide. Atomistic simulations carried out at Rice University show how this reaction happens. Image Credit: Evgeni Penev.
Iron (blue) can react with trace amounts of water to produce corrosive chemicals despite being bathed in “inert” supercritical fluids of carbon dioxide. Atomistic simulations carried out at Rice University show how this reaction happens. Image Credit: Evgeni Penev.
The reason behind this mechanism was unknown to materials scientists, but a research team at Rice University has come up with a theory that could add new strategies to safeguard iron from the surrounding.
Materials theorist Boris Yakobson and his collaborators at Rice’s George R. Brown School of Engineering have discovered through atom-level simulations that iron itself plays a role in its corrosion while being exposed to supercritical CO2 (sCO2) and trace amounts of water by encouraging the development of reactive species in the fluid that come back to attack it.
In their study, the researchers came to the conclusion that thin hydrophobic layers of 2D materials similar to graphene or hexagonal boron nitride can be employed as an obstacle between iron atoms and the reactive elements of sCO2. The study was published in the Cell Press journal Matter.
Rice graduate student Qin-Kun Li and research scientist Alex Kutana are co-lead authors of the study. Rice assistant research professor Evgeni Penev is a co-author.
Supercritical fluids are materials at a pressure and temperature that retains them approximately between phases — not all liquid, but not yet all gas. The properties of sCO2 make it a perfect working fluid because the scientists feel that it is affordable, noncorrosive and “essentially inert.”
Eliminating corrosion is a constant challenge, and it’s on a lot of people’s minds right now as the government prepares to invest heavily in infrastructure. Iron is a pillar of infrastructure from ancient times, but only now are we able to get an atomistic understanding of how it corrodes.
Boris Yakobson, Karl F. Hasselmann Professor, Materials Science and NanoEngineering, Rice University
Boris Yakobson is also a professor of chemistry.
The simulations performed in the Rice laboratory disclose the devil’s in the detail. Earlier studies have attributed corrosion to the existence of bulk water and other contaminants in the superfluid, but that is not essentially the case, stated Yakobson.
Water, as the primary impurity in sCO2, provides a hydrogen bond network to trigger interfacial reactions with CO2 and other impurities like nitrous oxide and to form corrosive acid detrimental to iron.
Qin-Kun Li, Study Co-Lead Author and Graduate Student, Rice University
Furthermore, the simulations displayed that the iron itself served as a catalyst, thereby reducing the reaction energy barriers at the interface present between iron and sCO2. Eventually, this results in the creation of a host of corrosive species: hydroxide, oxygen, nitrous acid and carboxylic acid.
The study demonstrates the power of theoretical modeling to resolve complex chemistry problems and it predicts thermodynamic reactions and estimates corrosion rates at the interface between sCO2 and iron. Also, the researchers displayed that all bets are off if there is more than a trace of water present in the superfluid, expediting corrosion.
The study was financially supported by the U.S. Department of Energy’s Fossil Energy Program, Division of Crosscutting R&D and Systems Integration (DE-AC05-00OR22725), through UT-Battelle LLC (4000174979).
Journal Reference:
Li, Q.-K., et al. (2021) Iron corrosion in the “inert” supercritical CO2, ab initio dynamics insights: How impurities matter. Matter. doi.org/10.1016/j.matt.2021.12.019.