.jpg) By Susha Cheriyedath, M.Sc.Reviewed by Skyla BailyJan 25 2022
By Susha Cheriyedath, M.Sc.Reviewed by Skyla BailyJan 25 2022A team of researchers recently published a paper in the journal Materials that demonstrated the significance of Aloe vera and the reaction temperature in promoting the crosslinking reaction during the synthesis of hydrogels.

Study: Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties. Image Credit: NUM LPPHOTO/Shutterstock.com
Hydrogels and Their Preparation
Natural polymer-based hydrogels are used extensively in pharmaceutical, cosmetic, and medical applications. Hydrogels can be characterized in terms of polarity, physical structure, and composition. Natural polymers such as gelatin, alginate, and collagen are typically used for preparing hydrogels.
Hydrogels are mainly prepared by chemical crosslinking or physical crosslinking, depending on the potential applications of the synthesized hydrogels. The final properties of hydrogel products primarily depend on the crosslinking agents, polymers, and monomers used for synthesizing the product.
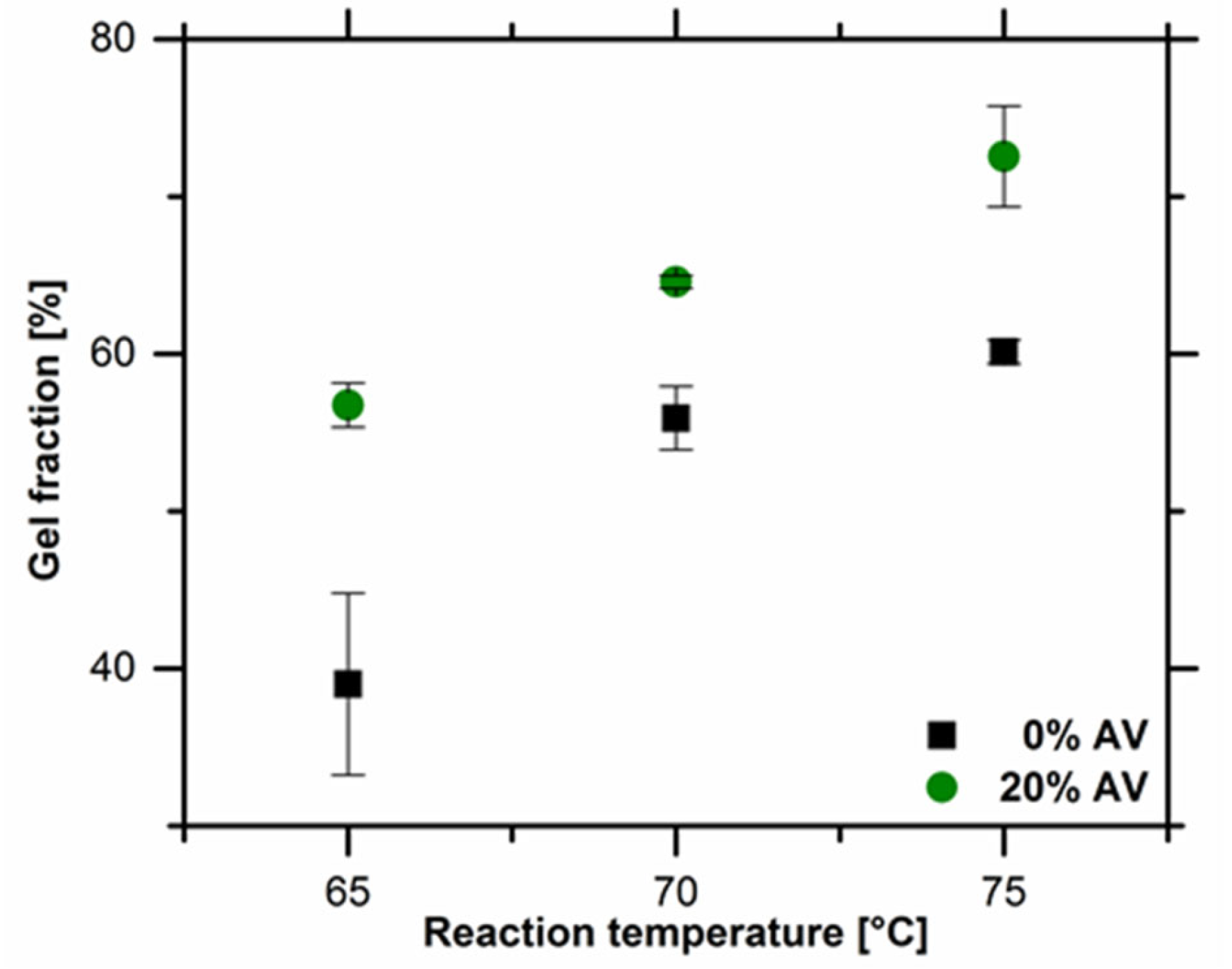
The gel fraction of obtained hydrogel materials. Image Credit: Bialik-W˛as, K et al., Materials
The Study
In this study, researchers intended to investigate a hydrogel system with good biodegradability, biocompatibility, and drug delivery properties. They selected sodium alginate as the natural polymer for synthesizing the proposed hydrogel system due to its non-toxicity, biodegradability, and extensive availability.
Poly(ethylene glycol)diacrylate (PEGDA) was used as the crosslinking agent, while poly(vinyl alcohol) (PVA) was utilized to provide better mechanical properties to the hydrogel system. Additionally, Aloe vera extract was utilized as a natural healing agent, while glycerin was used to improve the transdermal permeability of active substances such as proteins, enzymes, and antibiotics.
Ammonium persulfate was employed as an initiator. The reaction temperature in the study was kept low in order to reduce the potential loss of the biological properties of active substances. The effect of the reaction temperature on the degree of the swelling ratio and crosslinking of the synthesized hydrogels was monitored.
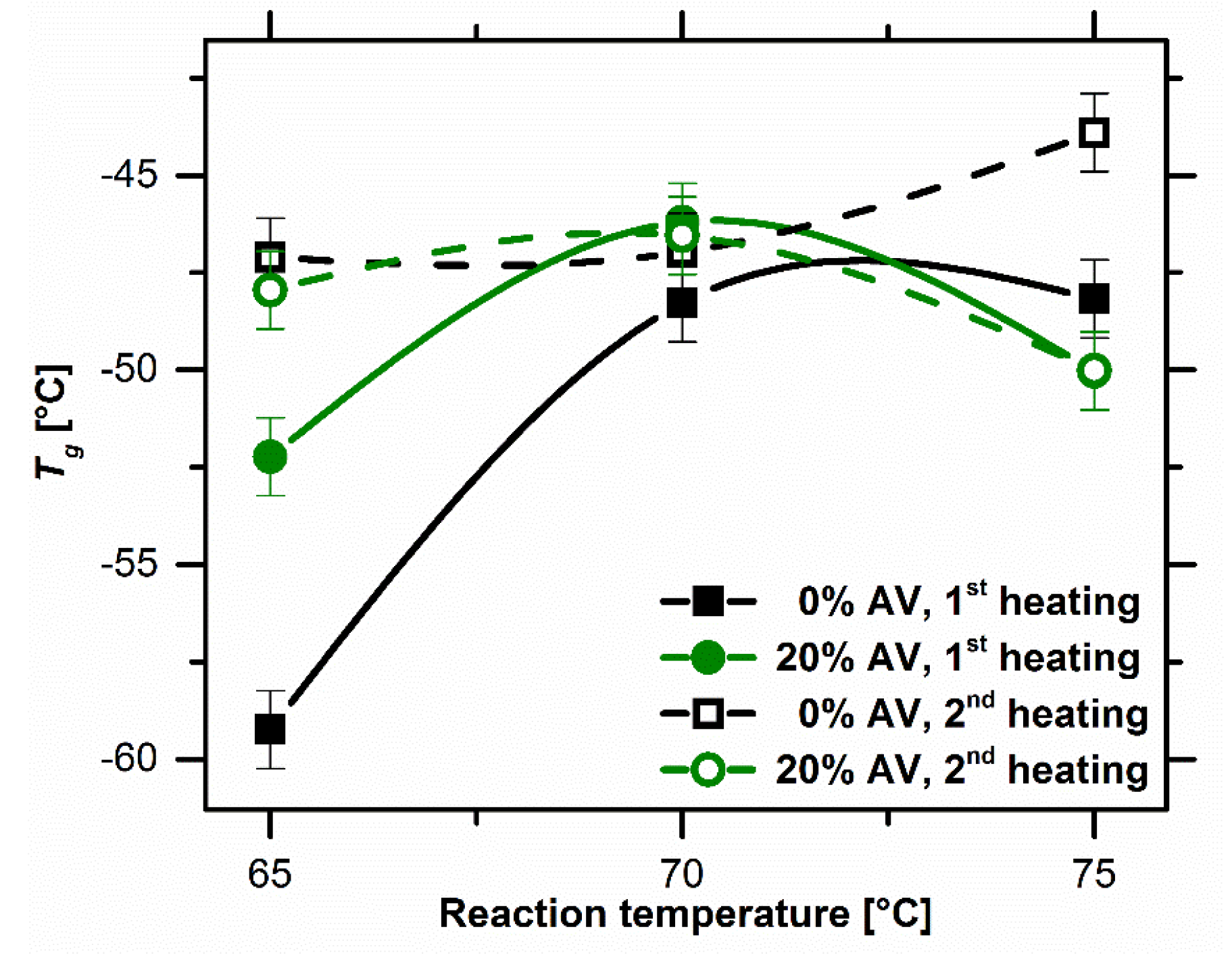
Glass transition temperature as a function of reaction temperature. Image Credit: Bialik-W˛as, K et al., Materials
Additionally, the thermal transitions of the final hydrogels and the substrates were also investigated in order to determine the final properties of the hydrogels that were influenced by the reaction temperature.
Stock aqueous solutions were prepared for obtaining the hydrogel materials. In the case of Aloe vera modified materials, an Aloe vera stock solution of 20% v/v was added. Eventually, these materials were poured into Petri dishes and then placed on a heating plate at three temperatures – 65 oC, 70 oC, and 75 oC for 90 minutes, followed by storage at ambient conditions for 24 hours.
For determining the gel fraction, three 10 × 10 mm pieces were initially cut from each hydrogel, conditioned for 24 h at 40 oC, and weighed. Then, those pieces were submerged in the distilled water for 48 h at ambient conditions, conditioned for 24 h at 40 oC, and again weighed. The swelling ratio was calculated by immersing the samples in distilled water and phosphate buffer solution (PBS) in ambient temperature conditions.
A Fourier transform infrared (FT-IR) spectrometer with an iD7 ATR accessory in the range of 4000−400 cm-1 was employed for studying the chemical structure of the hydrogels. The team also used a scanning electron microscope (SEM) with energy-dispersive X-ray spectroscopy (EDS) to analyze the samples. Differential scanning calorimetry (DSC) was used for investigating the thermal transitions of the hydrogels. All cooling/heating steps were performed at a rate of 10 K/min.
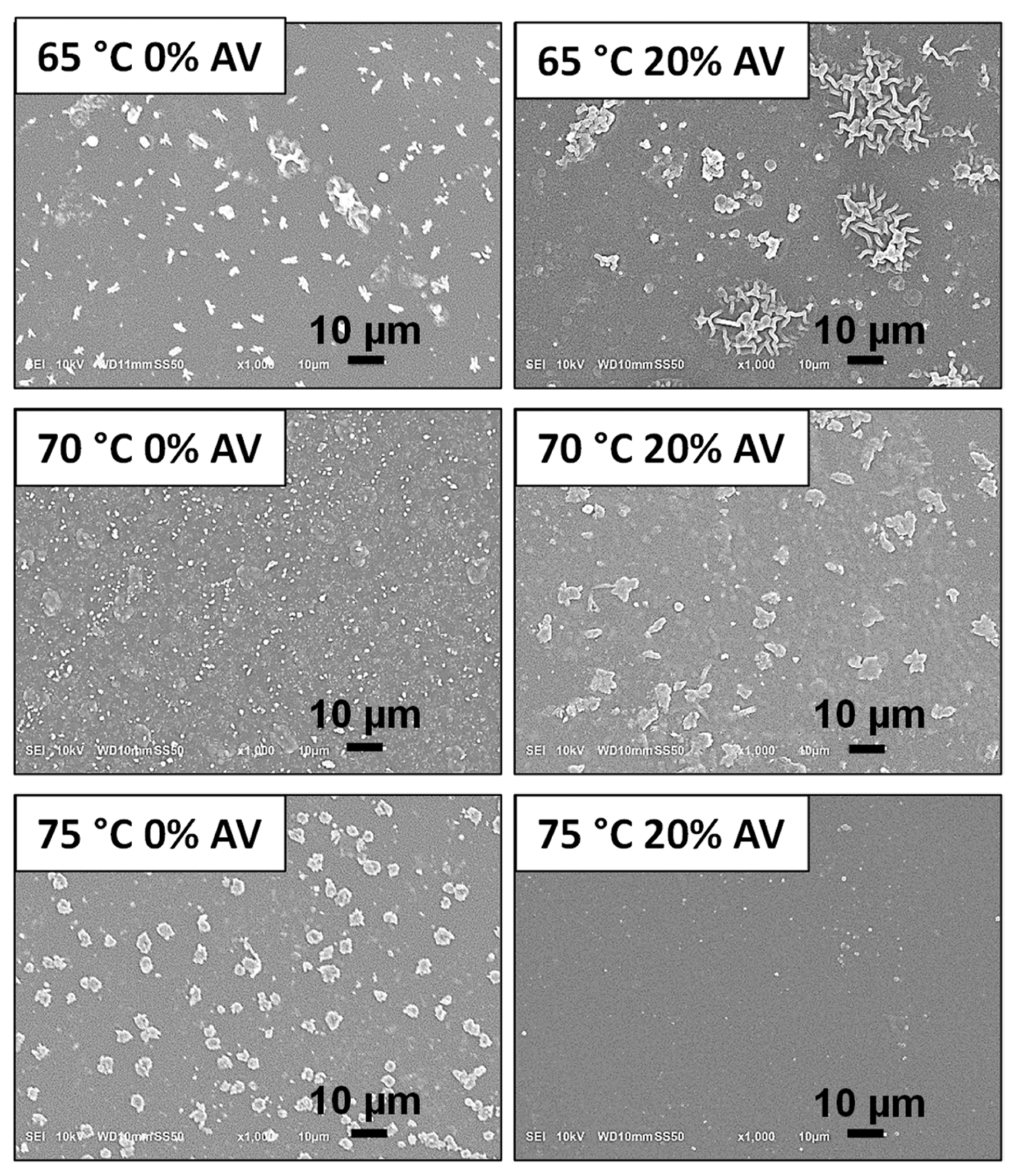
SEM micrographs presenting the morphology of obtained hydrogels before immersion in PBS solution. Image Credit: Bialik-W˛as, K et al., Materials
Observations
Lower values of gel fraction were observed in the samples without Aloe vera solution compared to the samples with Aloe vera solution due to the viscosity of the reaction mixture. The dependence of the gel fraction values on the concentration of Aloe vera was mainly attributed to the presence of additional mucopolysaccharides and polysaccharides in Aloe vera.
The value of gel fractions increased when the crosslinking temperature was increased from 65 oC to 75 oC, indicating the key role played by the reaction temperature in the degree of chemical crosslinking. Results from the swelling degrees showed no difference in the behavior of the tested hydrogel samples in both PBS solution and distilled water. However, in samples containing Aloe vera, the swelling ratio was slightly higher in water compared to PBS.
The swelling ratio in all hydrogel samples, including the samples containing Aloe vera, reached a maximum value at the initial stages, often within 15 minutes, and then decreased in a gradual manner. The lowest swelling abilities of the samples were observed at 65 oC. The FT-IR spectra showed that the vibrations from the -C=C bonds were less visible, and they shifted from their original positions during the crosslinking reaction of the samples containing Aloe vera at 75 oC, indicating higher values of gel fraction and a more complete crosslinking reaction.

SEM micrographs show the cross-section of obtained hydrogels. Image Credit: Bialik-W˛as, K et al., Materials
Observations from the DSC curves indicated that both samples attained an endothermic peak at 10 oC and a complex exothermic peak at 70 oC, associated with the beginning of the crosslinking reaction. The SEM images showed that the cross-section of the surface of the samples with Aloe vera was less porous and more compact compared to the samples without Aloe vera.
To summarize, results from gel fraction measurement, DSC, and FT-IR spectroscopy demonstrated that higher reaction temperature and inclusion of Aloe vera can promote the crosslinking reaction during the synthesis of a sodium alginate-based hydrogel.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Bialik-W˛as, K., Raftopoulos, K.N., Pielichowski, K. Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties. Materials 2022, 15, 748. https://www.mdpi.com/1996-1944/15/3/748/htm