In a recent study published in the journal Materials, researchers from Poland analyzed the flocculation properties of starch, chitosan, and oxidized derivatives used in water treatment.

Study: The Use of Chitosan and Starch-Based Flocculants for Filter Backwash Water Treatment. Image Credit: Nazriamani/Shutterstock.com
Issues with Traditional Water Treatment Systems
Water purification is an essential step in the production process of safe drinking water and is currently identified as a critical scientific issue. Filtration is the most common process in traditional water treatment systems, but it produces a large amount of filter backwash water (FBW) with colloidal contaminants that are washed out of the filter bed. To reduce wastewater production, FBW is generally treated using a technological process that uses coagulation and flocculation.
Coagulation is a phenomenon in which colloid particles are deposited due to the addition of electrolytes, such as aluminum or iron compounds, and the process of forming larger agglomerates is known as flocculation. They are used in wastewater treatment by developing well-defined aggregates that can rapidly settle out of the water, and these techniques effectively remove fine particles suspended in the liquid.
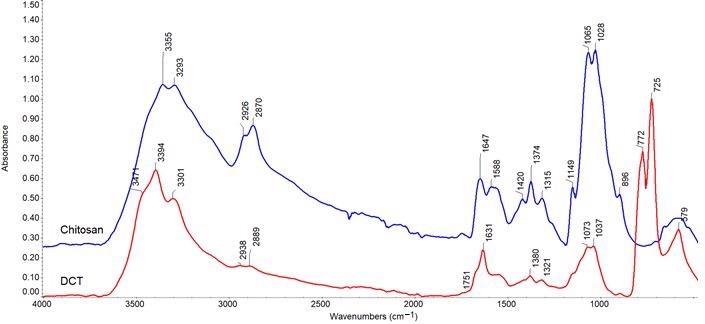
ATR-FTIR spectra for chitosan (blue) and DCT (red). Image credit: Maćczak, P et al., Materials
In a recent study, researchers synthesized polysaccharide-based flocculants such as dialdehyde chitosan (DCT) and dialdehyde starch (DST) and tested their flocculation properties. The starch and chitosan derivatives were produced using periodate, and the obtained derivatives were characterized by various tests.
Methodology
The materials used in the current study were chitosan, starch from corn, acetic acid, acetone, and sodium periodate. A water treatment plant (WTP) in Kutno, Poland, was used to collect water samples. Oxidized polysaccharides having hydrolyzed polyaluminum chloride were used as a primary coagulant.
For the study, researchers selected eight commonly used polyacrylamide flocculants with different structures. Oxidation with sodium periodate produced the dialdehyde derivatives of starch (DST) and chitosan (DCT) (NaIO4); DST and DCT were used for filter backwash water treatment.
Additionally, thermogravimetric analysis (TGA), digital jar test, scanning electron microscope (SEM) analysis, and Fourier transform infrared spectroscopy (FTIR) analysis were conducted. The microorganisms' biochemical oxygen demand (BOD) was measured using the respirometric method to determine microbes' metabolic activity.
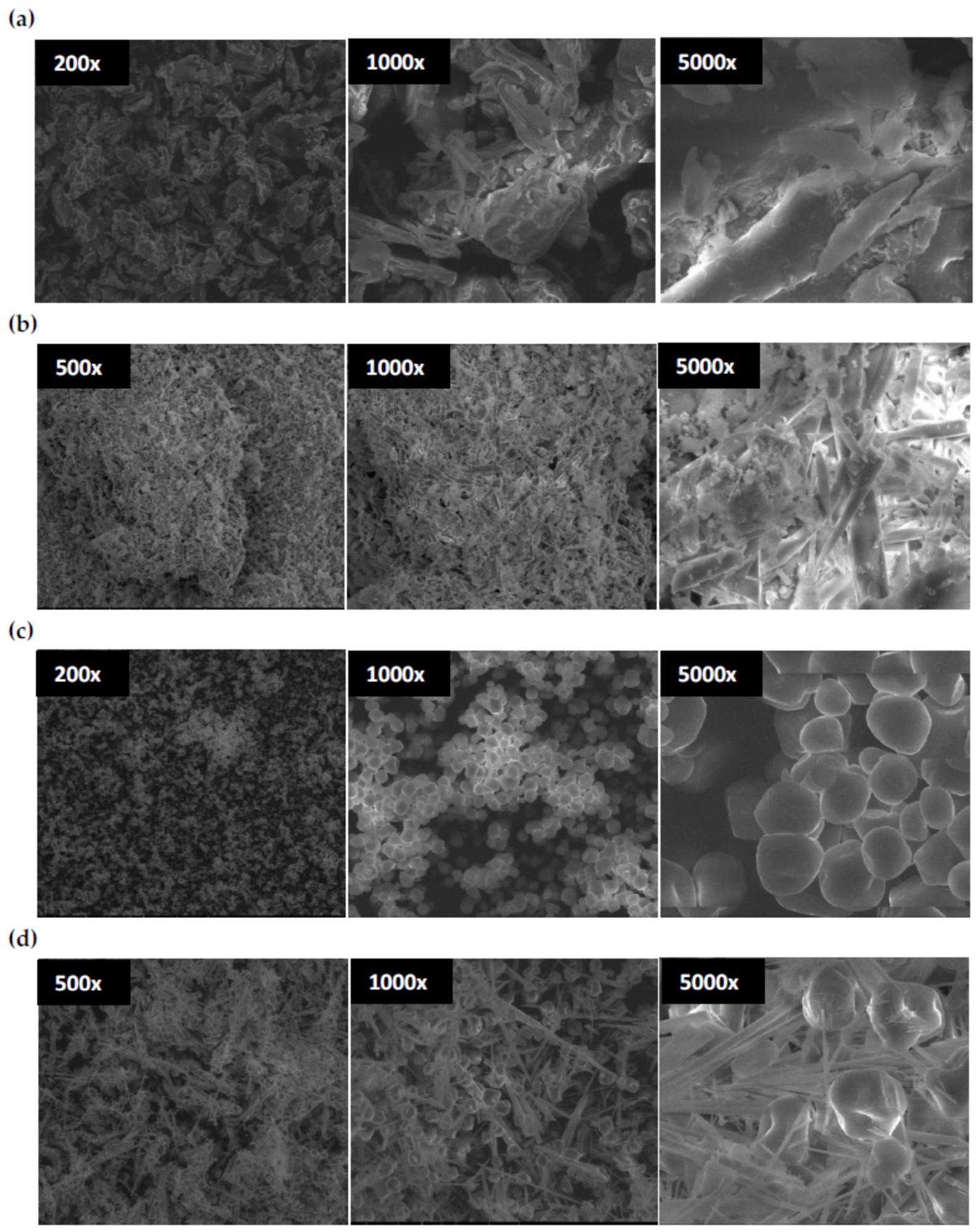
SEM images of chitosan (a), dialdehyde chitosan (b), corn starch (c), dialdehyde starch, (d) at different magnification. Image credit: Maćczak, P et al., Materials
Results
FTIR spectroscopy confirmed that the structural changes in the samples were caused by the oxidation of chitosan and corn starch.
The SEM image of native chitosan showed irregularly shaped aggregates (20 to 200 µm) and similarities with the shape of thick, elongated sticks. Further, the oxidation of chitosan resulted in significant morphological changes, with objects of smaller size appearing in the DCT image.
It was also observed that oxidation of the polysaccharides caused morphological changes and variations in their physicochemical properties, improved reactivity, and solubility, and decreased macromolecule order and thermal stability.
TGA results indicated that fragmentation, accompanied by the release of low-molecular-weight products, was more efficient in DCT and DST than in unoxidized chitosan (CT) and starch (ST). Moreover, thermal crosslinking was ineffective in DST, where 100% weight loss occurred because of incomplete decomposition. The BOD test indicated that oxygen demand increased over 11 and 8 times after 14 days of test for chitosan and DCT, respectively.
The turbidity and iron removal efficiency results based on synthetic flocculant dose showed that all polymers tested by researchers in the first jar test series had iron concentrations and turbidity reductions of over 90%. Additionally, the findings show that functional groups perform an important role in the flocculation mechanism.
Chitosan produced optimum results due to reactive amine groups in macromolecules and its capability of binding metal ions. Moreover, the presence of amino groups in chitosan gives it cationic properties, which support electrostatic interactions of the removed pollutants.
Furthermore, complex iron ions and other positively charged impurities reacted with the remaining acetylamino in CT and produced aldehyde groups in DCT and DST. The formation of hydrogen bonds by hydroxyls in chitosan also affects the aggregation process. Additionally, in the case of polysaccharides, specific chemical reactions between the reactive groups of the polymer and the contaminant particle may also have affected the process.

Jar test with chitozan-based flocculant in polymer dose (a) 0.1, (b) 0.2, (c) 0.3, (d) 0.5, (e) 1.0, (f) 0.2 mg/L + 1 mg Al3+/L of PAX XL10 coagulant, respectively. Image credit: Maćczak, P et al., Materials
Conclusions
Researchers of the current study demonstrated the successful replacement of commercial flocculants based on synthetic polymers with starch, chitosan, and dialdehyde derivatives. The research also indicated the optimal CT, DCT, ST, and DST doses that can be used for wastewater treatment. Moreover, unmodified chitosan was identified as the most effective flocculant as it removed turbidity and iron ions to the same level as commercial polyacrylamide flocculants.
The flocculants suggested by researchers are inexpensive and can be effectively used in water treatment. Hence, polysaccharide-based flocculants in the filtration of FBW have significant potential in wastewater treatment.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M.; Węgrzynowska-Drzymalska, K. The Use of Chitosan and Starch-Based Flocculants for Filter Backwash Water Treatment. Materials 2022, 15, 1056. https://www.mdpi.com/1996-1944/15/3/1056